Compartment-specific small non-coding RNA changes and nucleolar defects in human mesial temporal lobe epilepsy
- PMID: 39509000
- PMCID: PMC11543739
- DOI: 10.1007/s00401-024-02817-8
Compartment-specific small non-coding RNA changes and nucleolar defects in human mesial temporal lobe epilepsy
Abstract
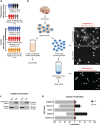
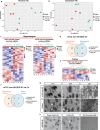
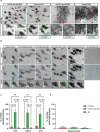
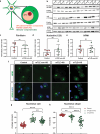
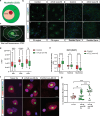
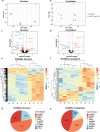
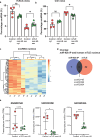
Mesial temporal lobe epilepsy (mTLE) is a debilitating disease characterized by recurrent seizures originating from temporal lobe structures such as the hippocampus. The pathogenic mechanisms underlying mTLE are incompletely understood but include changes in the expression of non-coding RNAs in affected brain regions. Previous work indicates that some of these changes may be selective to specific sub-cellular compartments, but the full extent of these changes and how these sub-cellular compartments themselves are affected remains largely unknown. Here, we performed small RNA sequencing (RNA-seq) of sub-cellular fractions of hippocampal tissue from mTLE patients and controls to determine nuclear and cytoplasmic expression levels of microRNAs (miRNAs). This showed differential expression of miRNAs and isomiRs, several of which displayed enriched nuclear expression in mTLE. Subsequent analysis of miR-92b, the most strongly deregulated miRNA in the nucleus, showed accumulation of this miRNA in the nucleolus in mTLE and association with snoRNAs. This prompted us to further study the nucleolus in human mTLE which uncovered several defects, such as altered nucleolar size or shape, mis-localization of nucleolar proteins, and deregulation of snoRNAs, indicative of nucleolar stress. In a rat model of epilepsy, nucleolar phenotypes were detected in the latency period before the onset of spontaneous seizures, suggesting that nucleolar changes may contribute to the development of seizures and mTLE. Overall, these data for the first time implicate nucleolar defects in the pathogenesis of mTLE and provide a valuable framework for further defining the functional consequences of altered sub-cellular RNA profiles in this disease.
Keywords: Epilepsy; Human; Non-coding RNA; Nucleolus; Small nucleolar RNA; microRNA.
© 2024. The Author(s).
Conflict of interest statement
M.T.V. is employed by the company Omiics ApS. The remaining authors declare no conflict of interest.
Figures

References
-
- Abs E, Goorden SMI, Schreiber J, Overwater IE, Hoogeveen-Westerveld M, Bruinsma CF et al (2013) TORC1-dependent epilepsy caused by acute biallelic Tsc1 deletion in adult mice. Ann Neurol 74:569–579. 10.1002/ana.23943 - PubMed
-
- Andersen JS, Lam YW, Leung AKL, Ong S-E, Lyon CE, Lamond AI et al (2005) Nucleolar proteome dynamics. Nature 433:77–83. 10.1038/nature03207 - PubMed
-
- Baltanás FC, Berciano MT, Tapia O, Narcis JO, Lafarga V, Díaz D et al (2019) Nucleolin reorganization and nucleolar stress in Purkinje cells of mutant PCD mice. Neurobiol Dis 127:312–322. 10.1016/J.NBD.2019.03.017 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

