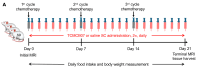
Melanocortin-4 receptor antagonist TCMCB07 alleviates chemotherapy-induced anorexia and weight loss in rats
- PMID: 39509261
- PMCID: PMC11684807
- DOI: 10.1172/JCI181305
Melanocortin-4 receptor antagonist TCMCB07 alleviates chemotherapy-induced anorexia and weight loss in rats
Abstract
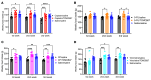
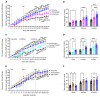
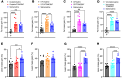
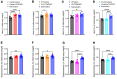
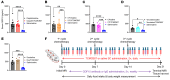
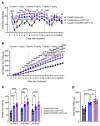
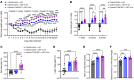
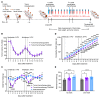
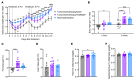
Cancer patients undergoing chemotherapy often experience anorexia and weight loss that substantially deteriorates overall health, reduces treatment tolerance and quality of life, and worsens oncologic outcomes. There are currently few effective therapeutic options to mitigate these side effects. The central melanocortin system, which plays a pivotal role in regulating appetite and energy homeostasis, presents a logical target for treating anorexia and weight loss. In this preclinical study, we evaluated the efficacy of TCMCB07, a synthetic antagonist of the melanocortin-4 receptor, in mitigating anorexia and weight loss in several rat models of chemotherapy: cisplatin, 5-fluorouracil, cyclophosphamide, vincristine, doxorubicin, and a combination of irinotecan and 5-fluorouracil. Our results indicate that peripheral administration of TCMCB07 improved appetite, stabilized body weight, preserved fat and heart mass, and slightly protected lean mass after multiple cycles of chemotherapy. Furthermore, combining TCMCB07 with a growth differentiation factor 15 antibody enhanced treatment effectiveness. Similar effects from TCMCB07 treatment were observed in a rat tumor model following combination chemotherapy. No notable adverse effects nor increased chemotherapy-related toxicities were observed with TCMCB07 treatment. These findings suggest that peripheral administration of TCMCB07 holds promise as a therapeutic approach for alleviating chemotherapy-induced anorexia and weight loss, potentially benefiting numerous patients undergoing chemotherapy.
Keywords: Cancer; Melanocortin; Oncology; Therapeutics.
Conflict of interest statement
Figures