3-nitropyridine analogues as novel microtubule-targeting agents
- PMID: 39509402
- PMCID: PMC11542830
- DOI: 10.1371/journal.pone.0307153
3-nitropyridine analogues as novel microtubule-targeting agents
Abstract
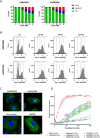
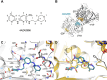
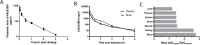
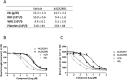
Microtubule-targeting agents are an important class of anti-cancer drugs; their full potential is however not realized because of significant myelotoxicity and neurotoxicity. We here report 3-nitropyridine analogues as a novel group of microtubule-targeting agents with potent anti-cancer effects against a broad range of cancer types. We show that these 3-nitropyridines induce cell cycle arrest in the G2-M phase and inhibit tubulin polymerization by interacting with tubulin. Determination of the tubulin-4AZA2996 structure by X-ray crystallography demonstrated that this class of compounds binds to the colchicine-site of tubulin. Furthermore, the anti-cancer effect was demonstrated both in vitro and in vivo in a murine heterotopic xenograft model of colon cancer. When administered intravenously, 4AZA2891 effectively inhibited cancer growth. Whereas 3-nitropyridine compounds do not induce myelotoxicity at pharmacological doses, the neurotoxicity associated with microtubule-targeting agents is still present.
Copyright: © 2024 Herman et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Jordan MA, Kamath K. How do microtubule-targeted drugs work? An overview. Curr Cancer Drug Targets. 2007;7: 730–42. Available: https://pubmed.ncbi.nlm.nih.gov/18220533/ doi: 10.2174/156800907783220417 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

