Consent-Related Outcomes in the Alteplase Compared to Tenecteplase Trial
- PMID: 39509663
- PMCID: PMC11558543
- DOI: 10.1212/WNL.0000000000209974
Consent-Related Outcomes in the Alteplase Compared to Tenecteplase Trial
Abstract
Background and objectives: In recent years, researchers have sought to address the challenges of obtaining informed consent for participation in acute stroke trials. We studied outcomes related to the use of deferral of consent in the phase 3 Alteplase Compared to Tenecteplase (AcT) trial.
Methods: As part of our protocol, we captured methods of consent, participant withdrawals, door-to-randomization times, and door-to-needle times. Participants at 3 sites were invited to complete a survey of attitudes regarding consent for AcT and for acute stroke trials generally.
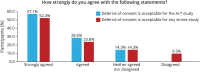
Results: The AcT trial enrolled 1,600 participants from 22 centers across Canada of whom 1,537 were enrolled through deferral of consent (96.0%) and 63 (4.0%) were enrolled by prospective verbal consent followed by written informed consent. Of those enrolled by deferral of consent, 95% (1,454/1,537) consented to ongoing participation. Door-to-randomization times were similar regardless of method of consent, with an overall median of 30 minutes (interquartile range [IQR] 22-42): 29 minutes (IQR 22-42) in the deferral of consent group vs 32 minutes (IQR 25-44) in the prospective consent group (p = 0.1602). Survey respondents overwhelming agreed or strongly agreed with the use of deferral of consent in AcT (86%) and in any acute stroke trial (76%).
Discussion: Deferral of consent was broadly acceptable to participants in the AcT trial as demonstrated by low rates of withdrawal and by survey results. Door-to-randomization times using deferral of consent in AcT were short, although a system of prospective verbal consent used at 1 center took only slightly longer. These results support the importance of innovation around consent for acute stroke trials.
Conflict of interest statement
The authors report no relevant disclosures. Go to
Figures
References
-
- Janssen PM, Chalos V, van den Berg SA, et al. ; MR CLEAN Registry Investigators. Neurological deficits in stroke patients that may impede the capacity to provide informed consent for endovascular treatment trials. J Stroke Cerebrovasc Dis. 2019;28(12):104447. doi:10.1016/j.jstrokecerebrovasdis.2019.104447 - DOI - PubMed
-
- Sajobi T, Singh N, Almekhlafi MA, et al. . Alteplase compared to tenecteplase in patients with acute ischemic stroke (AcT) trial: protocol for a pragmatic registry linked randomized clinical trial. Stroke: Vasc Interv Neurol. 2022:e12329. doi:10.1161/svin.121.000447 - DOI
-
- Menon BK, Buck BH, Singh N, et al. . Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet. 2022;400(10347):161-169. doi:10.1016/S0140-6736(22)01054-6 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials