Disease-free survival as surrogate for overall survival in real-world settings for esophageal cancer: an analysis of SEER-Medicare data
- PMID: 39510024
- PMCID: PMC11574803
- DOI: 10.1016/j.esmoop.2024.103934
Disease-free survival as surrogate for overall survival in real-world settings for esophageal cancer: an analysis of SEER-Medicare data
Abstract
Background: Establishing surrogate endpoints for overall survival (OS) may expedite assessment of new therapies in esophageal cancer (EC) and gastroesophageal junction cancer (GEJC). This study aimed to evaluate disease-free survival (DFS) as a surrogate endpoint for OS.
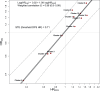
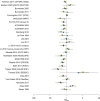
Methods: Patients from the Surveillance, Epidemiology, and End Results (SEER)-Medicare database aged ≥66 years with resection after primary diagnosis of stage 2 or 3 EC/GEJC between 2009 and 2017 were analyzed (N = 925; median follow-up 26.2 months). Surrogacy was assessed by evaluating individual level associations between DFS and OS using Spearman's rank correlation and the association between treatment effects by Pearson's correlation coefficient. To evaluate the association between treatment effects, patients were classified in synthetic clusters based on treatments received. Propensity score matching addressed imbalances in baseline characteristics between treatment and control groups in the clusters. Predictive performance of the surrogacy equation was assessed internally for the generated clusters via leave-one-out cross-validation and externally via predictions for 26 clinical trials of early-stage EC/GEJC.
Results: Patients were mostly male (84%), non-Hispanic white (89.3%), with median age 71.8 years, and cancer stages 2 (50.4%) and 3 (49.6%). Cancer types were adenocarcinoma (76.1%), squamous cell carcinoma (10.4%), and other types (13.5%). Most patients 766/925 (82.8%) received neoadjuvant therapy (680/766 chemoradiotherapy versus 86/766 chemotherapy alone) while 23.6% of the patients received adjuvant therapy. Within each treatment setting, most [705/766 (92.0%) of neoadjuvant therapy and 178/218 (81.7%) of adjuvant therapy] received multi-agent chemotherapy. The individual level correlation was 0.76 (95% confidence interval 0.70-0.80). The correlation between treatment effects was 0.96 (95% confidence interval 0.80-0.99) with a corresponding surrogate threshold effect of 0.71. Both internal (91%) and external (89%) validation of the model demonstrated high predictive accuracy.
Conclusions: Correlations between DFS and OS were meaningful at both individual and treatment effect level. The derived surrogacy equation enables reliable early assessments of OS benefit from the observed DFS benefit for early-stage EC/GEJC treatments in real-world settings.
Keywords: disease-free survival; esophageal cancer; gastroesophageal junction cancer; meta-analysis; overall survival; real-world data; surrogacy.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Siegel R.L., Giaquinto A.N., Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. - PubMed
-
- National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology: Esophageal and Esophagogastric Junction Cancers. V.3. 2024. https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf Available at.
-
- American Cancer Society Cancer facts & figures. 2024. https://www.cancer.org/cancer/types/esophagus-cancer/detection-diagnosis... Available at.
-
- Zeng H., Zheng R., Guo Y., et al. Cancer survival in China, 2003-2005: a population-based study. Int J Cancer. 2015;136(8):1921–1930. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

