Versican binds collagen via its G3 domain and regulates the organization and mechanics of collagenous matrices
- PMID: 39510178
- PMCID: PMC11626796
- DOI: 10.1016/j.jbc.2024.107968
Versican binds collagen via its G3 domain and regulates the organization and mechanics of collagenous matrices
Abstract
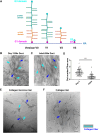
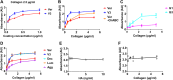
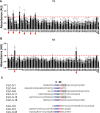
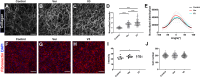
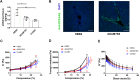
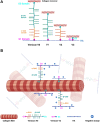
Type I collagen is the most abundant structural protein in the body and, with other fibrillar collagens, forms the fibrous network of the extracellular matrix. Another group of extracellular matrix polymers, the glycosaminoglycans, and glycosaminoglycan-modified proteoglycans, play important roles in regulating collagen behaviors and contribute to the compositional, structural, and mechanical complexity of the extracellular matrix. While the binding between collagen and small leucine-rich proteoglycans has been studied in detail, the interactions between collagen and the large bottlebrush proteoglycan versican are not well understood. Here, we report that versican binds collagen directly and regulates collagen structure and mechanics. Versican colocalizes with collagen fibers in vivo and binds to collagen via its C-terminal G3 domain (a non-GAG-modified domain present in all known versican isoforms) in vitro; it promotes the deposition of a highly aligned collagen-rich matrix by fibroblasts. Versican also shows an unexpected effect on the rheology of collagen gels in vitro, causing decreased stiffness and attenuated shear strain stiffening, and the cleavage of versican in the liver results in reduced tissue compression stiffening. Thus, versican is an important collagen-binding partner and plays a role in modulating collagen organization and mechanics.
Keywords: collagen Ligands Collection; glycosaminoglycan; hyaluronic acid; liver rheology; versikine.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests. Arkadiusz Bonna and Richard W. Farndale are former employees of CambCol Laboratories and are now directors of Triple Helical Peptides Ltd. The other authors declare they have no conflicts of interest with the contents of this article.
Figures

References
-
- Kalamajski S., Oldberg Å. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol. 2010;29:248–253. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

