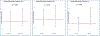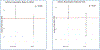Modification of asthma treatment efficacy by healthcare access: A reanalysis of AsthmaNet Step-Up Yellow Zone Inhaled Corticosteroids to Prevent Exacerbations (STICS) clinical trial
- PMID: 39510322
- PMCID: PMC11960804
- DOI: 10.1016/j.rmed.2024.107853
Modification of asthma treatment efficacy by healthcare access: A reanalysis of AsthmaNet Step-Up Yellow Zone Inhaled Corticosteroids to Prevent Exacerbations (STICS) clinical trial
Abstract
Background: While randomized controlled trials (RCTs) in asthma management are designed to balance known and unknown variables across treatment groups, including social and environmental co-exposures, it remains important to consider how these co-exposures influence disease progression and treatment outcomes. The importance of considering socio-environmental co-exposures in the context of asthma is twofold: 1) asthma disproportionately affects low-income urban communities, where air pollution and chronic stress are pervasive; and 2) despite the wide range of asthma treatments, inadequate disease control persists.
Methods: In the present ancillary study of the Step-Up Yellow Zone Inhaled Corticosteroids to Prevent Exacerbations (STICS) RCT, we investigated how socio-environmental factors, such as air pollution exposure and healthcare access, modify the effect of inhaled corticosteroid (ICS) therapy in children with asthma. The original STICS RCT evaluated the efficacy and safety of increasing the dose of inhaled glucocorticoids from a baseline daily low dose to five times the daily dose for 7 days in school-age children with mild -to-moderate persistent asthma who began to have short-term loss of asthma control (Jackson et al., 2018 Mar 8) [1]. Our study adds onto those findings by incorporating residential level particulate matter 2.5 μg/m3 (PM2.5) and geographic health provider shortage areas (HPSA) as potential modifiers.
Results: Consistent with the main trial results, we did not find a difference in the number of exacerbations between treatment arms. However, we found the effect of receiving 5xICS, as compared with 1xICS on the time to prednisone was significantly different for children living in areas a shortage of health professionals (HR: 2.09; 95 % CI: 0.74, 5.95) than for children living in no shortage areas (HR: 0.40; 95 % CI: 0.21, 0.77).
Conclusion: This finding underscores the importance of considering environmental and social factors in asthma treatment.
Trial registration: ClinicalTrials.gov ID NCT02066129 https://clinicaltrials.gov/study/NCT02066129.
Keywords: ICS; Pediatric asthma; RCT; SDOH.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures