Patient-centred composite scores as tools for assessment of response to biological therapy for paediatric and adult severe asthma
- PMID: 39510551
- PMCID: PMC11948419
- DOI: 10.1183/13993003.00691-2024
Patient-centred composite scores as tools for assessment of response to biological therapy for paediatric and adult severe asthma
Abstract
Background: We have previously developed Core Outcome Measures sets for Severe Asthma (COMSA) by multi-stakeholder consensus. There are no patient-centred tools to quantify response to biological therapies for severe asthma. We aimed to develop paediatric and adult CompOsite iNdexes For Response in asthMa (CONFiRM) incorporating clinical parameters and patient-reported quality of life.
Methods: International expert healthcare professionals and patients with severe asthma were invited to 1) develop consensus levels of clinically relevant changes for each outcome measure within COMSA, 2) use multicriteria decision analysis to develop the CONFiRM scores and 3) assess their internal validity. A separate group of healthcare professionals evaluated CONFiRM's external validity.
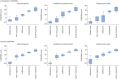
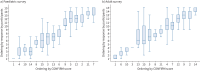
Results: Five levels of change for each COMSA outcome were agreed. Severe exacerbations and maintenance oral corticosteroid use were rated as the most important in determining both paediatric and adult CONFiRM scores. There was strong agreement between healthcare professionals and patients, although patients assigned greater importance to quality of life. The CONFiRM score quantified response to a biologic from -31 (deterioration) to 69 (best possible response). Paediatric and adult CONFiRMs had good discriminative ability for a sufficient (area under the curve ≥0.92) and a substantial (area under the curve ≥0.95) response to biologics. Both CONFiRMs demonstrated excellent external validity (Spearman correlation coefficients 0.9 and 0.8 for paediatric and adult, respectively; p<0.0001).
Conclusions: We have developed novel patient-centred paediatric and adult CONFiRMs that include quality of life measures. CONFiRMs should allow a more holistic understanding of response for the patient and a standardised assessment of the effectiveness of biologics between studies. Further research is needed to prospectively validate CONFiRM scores.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: E. Khaleva reports support for the present study from 3TR European Union IMI 2 and Asthma, Allergy and Inflammation Research (AAIR) Charity. C. Brightling reports support for the present study from 3TR and Leicester NIHR BRC; grants from GSK, AstraZeneca, Sanofi, Regeneron, Roche, Genentech, Novartis, Chiesi and Mologic; and consultancy fees from GSK, AstraZeneca, Sanofi, Regeneron, Roche, Genentech, Novartis, Chiesi and Mologic. T. Eiwegger reports grants from ALK and Greer Stallergen; consultancy fees from ALK; payment or honoraria for lectures, presentations, manuscript writing or educational events from Aimune, ThermoFisher, Nutricia/Danone, ALK and Novartis; payment for expert testimony from Aimune; participation on a data safety monitoring board or advisory board with ALK, Aimune and Nutricia/Danone; leadership roles with EAACI (Chair WG Biologicals and Board Immunology Section) and Allergy – European Journal of Allergy and Clinical Immunology (Associate Editor); and receipt of equipment, materials, drugs, medical writing, gifts or other services from MADX and Novartis. A. Altraja reports consultancy fees from AstraZeneca, Boehringer Ingelheim, GSK and Sanofi; payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, Berlin-Chemie Menarini, Boehringer Ingelheim, Norameda, GSK, Sanofi and Zentiva; payment for expert testimony from AstraZeneca, Boehringer Ingelheim, GSK and Sanofi; support for attending meetings from AstraZeneca, Berlin-Chemie Menarini, Boehringer Ingelheim and Norameda; participation on a data safety monitoring board or advisory board with AstraZeneca, Boehringer Ingelheim, GSK and Sanofi; and receipt of equipment, materials, drugs, medical writing, gifts or other services from Berlin-Chemie and Menarini. P. Bégin reports grants from Novartis, DBV Technologies, Sanofi and Regeneron; consultancy fees from Pfizer, Sanofi and DBV Technologies; and payment or honoraria for lectures, presentations, manuscript writing or educational events from ALK, Sanofi, Pfizer, AstraZeneca and Bausch Health. K. Blumchen reports grants from Novartis Pharma GmbH, Allergy Therapeutics, Aimmune Therapeutics, DBV Technologies and Hipp GmbH; consultancy fees from Novartis Pharma GmbH, Allergy Therapeutics, Aimmune Therapeutics and DBV Technologies; payment or honoraria for lectures, presentations, manuscript writing or educational events from Novartis Pharma GmbH, Allergy Therapeutics, Aimmune Therapeutics, DBV Technologies, ALK Bausch, Lomb, Allergopharma and ThermoFisher; support for attending meetings from DBV Technologies and Aimmune Therapeutics; participation on a data safety monitoring board or advisory board with Novartis Pharma GmbH, DBV Technologies and Aimmune Therapeutics; and a leadership role with West German Working Group for Pediatric Pneumology and Allergology (Board member). A. Bossios reports grants from AstraZeneca; payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, GSK, Novartis and Teva; support for attending meetings from Novartis; participation on a data safety monitoring board or advisory board with AstraZeneca, GSK, Novartis, Teva and Sanofi; and leadership roles with Severe Heterogeneous Asthma Research collaboration, Patient-centred (SHARP) (member of steering committee), Swedish National Airway Register (steering committee member), Nordic Severe Asthma Network (NSAN) (vice-chair), and ERS (Head of Assembly 5). A. Bourdin reports grants from AstraZeneca and Boehringer Ingelheim; consultancy fees from AstraZeneca, Boehringer Ingelheim, GSK, Novartis, Amgen, Regeneron, Sanofi, Chiesi and AB Science; payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, Boehringer Ingelheim, GSK, Novartis, Amgen, Regeneron, Sanofi, Chiesi and AB Science; support for attending meetings from AstraZeneca, Boehringer Ingelheim, GSK, Novartis, Amgen, Regeneron, Sanofi, Chiesi and AB Science; participation on a data safety monitoring board or advisory board with AB Science; and is a member of the GINA scientific committee. A. Ten Brinke reports grants from GSK, TEVA and AstraZeneca; payment or honoraria for lectures, presentations, manuscript writing or educational events from GSK, TEVA, AstraZeneca and Sanofi; participation on a data safety monitoring board or advisory board with GSK, Sanofi, TEVA, AstraZeneca, Boehringer Ingelheim and Sterna; and leadership roles with the Dutch Severe Asthma Registry: RAPSODI (Chair) and ERS CRC SHARP (National Lead for the Netherlands). G. Brusselle reports payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis and Sanofi. R.S. Bumbacea reports grants from AstraZeneca; consultancy fees from AstraZeneca, Chiesi and Novartis; payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, Chiesi and Novartis; support for attending meetings from AstraZeneca; participation on a data safety monitoring board or advisory board with AstraZeneca and Novartis; and a leadership role with Romanian Society of Allergy and Clinical Immunology (past president). T.B. Casale reports consultancy fees from Genentech, Novartis, Sanofi and Regeneron; and payment or honoraria for lectures, presentations, manuscript writing or educational events from Sanofi and GSK. G.W. Clarke reports support for the present study from AstraZeneca (employment, study funding, article processing charges, medical writing) and stock (or stock options) with AstraZeneca. R. Chaudhuri reports grants from AstraZeneca; payment or honoraria for lectures, presentations, manuscript writing or educational events from GSK, AstraZeneca, Teva, Chiesi and Sanofi; support for attending meetings from Chiesi and Sanofi; and participation on a data safety monitoring board or advisory board with GSK, AstraZeneca and Celltrion. K.F. Chung grants from the Medical Research Council (MRC), Engineering and Physical Sciences Research Council and GSK; payment or honoraria for lectures, presentations, manuscript writing or educational events from Novartis, AstraZeneca and Merck; and participation on a data safety monitoring board or advisory board with GSK, AstraZeneca, Novartis, Roche, Merck, Rickett-Beckinson, Nocion, Shionogi and Clean Breathing Institute (supported by Haleon). C. Coleman reports support for the present study from IMI (funding received to support this work by European Lung Foundation from European Commission's IMI 2 JU under grant agreement number 831434 (3TR)). J. Corren reports grants from AstraZeneca and Regeneron/Sanofi; consultancy fees from AstraZeneca and Regeneron/Sanofi; payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca and Regeneron/Sanofi; and stock (or stock options) with Allakos Pharmaceutical. S-E. Dahlén reports support for the present study from 3TR IMI; consultancy fees from AstraZeneca, Cayman Co, GSK, Novartis, Regeneron, Sanofi and Teva; and payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca and Sanofi. A. Deschildre reports payment or honoraria for lectures, presentations, manuscript writing or educational events from Novartis, GSK, Sanofi, Regeneron, AstraZeneca, Aimmune Therapeutics, DBV Technologies, Nestle Health Science, ALK, Stallergènes-Greer and Viatris; support for attending meetings from Novartis, GSK, Sanofi, Regeneron, AstraZeneca, Aimmune Therapeutics, DBV Technologies, Nestle Health Science, ALK, Stallergènes-Greer and Viatris; and participation on a data safety monitoring board or advisory board with Novartis, GSK, Sanofi, Regeneron, AstraZeneca, Aimmune Therapeutics, DBV Technologies, Nestle Health Science, ALK, Stallergènes-Greer and Viatris. R. Djukanovic reports support for the present study from ERS, TEVA, GSK, Novartis, Sanofi and Chiesi; consultancy fees from Synairgen; payment or honoraria for lectures, presentations, manuscript writing or educational events from GSK; participation on a data safety monitoring board or advisory board with Kymab (Cambridge); and stock (or stock options) with Synairgen. A. Exley reports stock (or stock options) with GSK (belonging to family). L. Fleming reports consultancy fees from Sanofi/Regeneron and AstraZeneca; and payment or honoraria for lectures, presentations, manuscript writing or educational events from Novartis and AstraZeneca. E.A. Gaillard reports grants from Gilead, Chiesi and Adherium and Helicon Health; payment or honoraria for lectures, presentations, manuscript writing or educational events from Circassia and Sanofi; support for attending meetings from Sanofi; leadership role with ERS (Secretary of Group 07.02); and receipt of equipment, materials, drugs, medical writing, gifts or other services from Propeller Health. M. Gappa reports payment or honoraria for lectures, presentations, manuscript writing or educational events from Aimmune, ALK, Berlin Chemie, GSK, HAL Allergy, Nestle, Novartis, Omron and Sanofi/Regeneron; and participation on a data safety monitoring board or advisory board with Aimmune, ALK, GSK, Nestle, Novartis and Sanofi. A. Gupta reports grants from GSK; and payment or honoraria for lectures, presentations, manuscript writing or educational events from Boehringer Ingelheim, GSK, Novartis and AstraZeneca. L.G. Heaney reports grants from AstraZeneca, Medimmune, Novartis UK, Roche/Genentech and GSK; payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, Novartis, Roche/Genentech, Sanofi, Circassia, GSK, Chiesi and Teva; support for attending meetings from AstraZeneca, Chiesi, Novartis, Boehringer Ingelheim, Sanofi, Teva and GSK; and participation on a data safety monitoring board or advisory board with AstraZeneca, Novartis, GSK, Chiesi, Teva, Theravance and Vectura. M. Henderson reports grants from Novartis, Pfizer, AstraZeneca, Chiesi Farmaceutici, GSK, AbbVie, LeoPharma, Boehringer Ingelheim, Sanofi, Regeneron, OM Pharma, MSD, Roche and DBV Technologies; and support for attending meetings from Novartis. D.J. Jackson reports grants from AstraZeneca; consultancy fees from AstraZeneca, GSK and Sanofi Regeneron; and payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, GSK and Sanofi Regeneron. B. Karadag reports payment or honoraria for lectures, presentations, manuscript writing or educational events from Vem Ilac, Sandoz and GSK; and a leadership role with GSK Turkey Severe Asthma Advisory Group. M.S. Koh reports grants from AstraZeneca; and payment or honoraria for lectures, presentations, manuscript writing or educational events from GSK, AstraZeneca, Novartis, Sanofi and Boehringer Ingelheim. M.V. Kopp reports grants from Bundesministerium für Bildung & Forschung (BMBF) (Grant für das Deutsche Zentrum für Lungenfroschung); consultancy fees from Allergopharma GmbH and Sanofi Aventis GmbH; payment or honoraria for lectures, presentations, manuscript writing or educational events from Allergopharma GmbH, Sanofi Aventis GmbH, ALKAbello, Chiesi GmbH, Infectopharm GmbH, Novartis Pharma GmbH and Vertex GmbH; participation on a data safety monitoring board or advisory board with Allergopharma GmbH, and a leadership role with Society “Gesellschaft für Pädiatrische Pneumologie” GPP e.V. (past president). G.H. Koppelman reports grants from Netherlands Lung Foundation, European Union, Ubbo Emmius Foundation, GSK, Vertex, TEVA the Netherlands and Zon MW (VICI grant); consultancy fees from GSK, Pure IMS and AstraZeneca; and payment or honoraria for lectures, presentations, manuscript writing or educational events from Sanofi, Boehringer Ingelheim and AstraZeneca. E. Melén reports consultancy fees from ALK, AstraZeneca, Chiesi, Novartis and Sanofi. K. Milger reports grants from BMBF, Stiftung Atemweg, Janssen, Novartis and AstraZeneca; consultancy fees from AstraZeneca, GSK, Janssen and Sanofi; payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, GSK, Sanofi, Janssen and Novartis; and leadership roles with DGP Section allergy and immunology (Vice Chair) and German Asthma Net eV (Scientific Advisory Board). A. Moeller reports grants from Vertex Inc.; consultancy fees from Vertex Inc.; payment or honoraria for lectures, presentations, manuscript writing or educational events from Vertex Inc.; participation on a data safety monitoring board or advisory board with Vertex Inc.; and a leadership role with ERS (Head of Assembly 7). C.S. Murray reports grants from NIHR, Asthma UK and MRC; payment or honoraria for lectures, presentations, manuscript writing or educational events from GSK, Novartis and ThermoFisher; and support for attending meetings from Sanofi. P. Nagakumar reports grants from NIHR; consultancy fees from Sanofi and AstraZeneca; and payment or honoraria for lectures, presentations, manuscript writing or educational events from GSK and Novartis. P. Nair reports grants from AstraZeneca, Teva, Sanofi, Foresee and Cyclomedica; consultancy fees from Arrowhead Pharma; payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, Teva and GSK; and participation on a data safety monitoring board or advisory board with Sanofi, Equillium and GSK. A. Nieto reports consultancy fees from ASAC Pharma; payment or honoraria for lectures, presentations, manuscript writing or educational events from Novartis, Immunotek and Uriach; and support for attending meetings from Immunotex. N.G. Papadopoulos reports grants from Capricare, Nestle, Numil and Vianex; and consultancy fees from Abbott, Abbvie, AstraZeneca, GSK, HAL, Medscape, Menarini/Faes Farma, Mylan, Novartis, Nutricia, OM Pharma and Regeneron/Sanofi. M.W. Pijnenburg reports consultancy fees from Sanofi; payment or honoraria for lectures, presentations, manuscript writing or educational events from Novartis and Abbvie; support for attending meetings from ERS; and a leadership role with ERS (Head of Assembly 7). K.C. Pike reports consultancy fees from Respiri and Adherium; payment or honoraria for lectures, presentations, manuscript writing or educational events from Sanofi; and support for attending meetings from GSK. C. Porsbjerg reports grants from AstraZeneca, GSK, Novartis, TEVA, Sanofi, Chiesi and ALK; consultancy fees from AstraZeneca, GSK, Novartis, TEVA, Sanofi, Chiesi and ALK; payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, GSK, Novartis, TEVA, Sanofi, Chiesi and ALK; and participation on a data safety monitoring board or advisory board with AstraZeneca, Novartis, TEVA, Sanofi and ALK. A. Rattu reports support for the present study from 3TR European Union IMI 2. H. Rupani reports grants from GSK and AstraZeneca; payment or honoraria for lectures, presentations, manuscript writing or educational events from GSK, AstraZeneca and Chiesi; and support for attending meetings from AstraZeneca. P. Seddon reports grants from NIHR; participation on a data safety monitoring board or advisory board with WAVE Study (sponsor and funder Inspiration Healthcare Limited); and receipt of equipment, materials, drugs, medical writing, gifts or other services from Vapotherm Inc. S. Siddiqui reports payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, GSK, Chiesi, WebMD, Areteia Therapeutics; participation on a data safety monitoring board or advisory board with AstraZeneca, GSK, Chiesi, WebMD, Areteia Therapeutics and MRC; leadership role with ERS Science Council; and stock (or stock options) with Eupnoos Ltd (co-founder and holds a 5% equity stake). F. Singer reports grants from Medical University of Graz and LungenLiga Bern; payment or honoraria for lectures, presentations, manuscript writing or educational events from Novartis Pharma Switzerland, Vertex Pharmaceuticals Switzerland and Vertex Pharmaceuticals Austria; and support for attending meetings from Chiesi Pharmaceuticals Austria. J.W. Upham reports payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, GSK and Sanofi; and a leadership role with Thoracic Society of Australia & New Zealand (president). S.J.H. Vijverberg reports grants from Lung Foundation Netherlands, ERA-PerMed and ZonMW; and leadership role with ERS (ECMC member). P.A.B. Wark reports a leadership role with National Asthma Council of Australia. M.E. Wechsler reports consultancy fees from Amgen, Areteia Therapeutics, AstraZeneca, Avalo Therapeutics, Boehringer Ingelheim, Celldex Cellergy Pharma, Cerecor, Cytoreason, Eli Lilly, Equillium, GSK, Incyte, Kinaset, Merck, Novartis, Om Pharma, Overtone Therapeutics/Foresite Labs, Phylaxis, Pulmatrix, Rapt Therapeutics, Regeneron, Roche/Genentech, Sanofi/Genzyme, Sentien, Sound Biologics, Tetherex Pharmaceuticals, Teva, Upstream Bio and Verona Pharma; payment or honoraria for lectures, presentations, manuscript writing or educational events from Amgen, Areteia Therapeutics, AstraZeneca, Avalo Therapeutics, Boehringer Ingelheim, Celldex Cellergy Pharma, Cerecor, Cytoreason, Eli Lilly, Equillium, GSK, Incyte, Kinaset, Merck, Novartis, Om Pharma, Overtone Therapeutics/Foresite Labs, Phylaxis, Pulmatrix, Rapt Therapeutics, Regeneron, Roche/Genentech, Sanofi/Genzyme, Sentien, Sound Biologics, Tetherex Pharmaceuticals, Teva, Upstream Bio and Verona Pharma; and participation on a data safety monitoring board or advisory board with Sentien. V. Yasinska reports participation on a data safety monitoring board or advisory board with AstraZeneca and GSK. G. Roberts reports support for the present study from EU IMI, and consultancy fees from AstraZeneca. The remaining authors have no potential conflicts of interest to disclose.
Figures





Comment in
-
Advancing patient-centred care in measuring response to biologics in severe asthma.Eur Respir J. 2025 Mar 27;65(3):2402113. doi: 10.1183/13993003.02113-2024. Print 2025 Mar. Eur Respir J. 2025. PMID: 40147860 No abstract available.
