Repetitive Sensory Stimulation Potentiates and Recruits Sensory-Evoked Cortical Population Activity
- PMID: 39510832
- PMCID: PMC11756624
- DOI: 10.1523/JNEUROSCI.2189-23.2024
Repetitive Sensory Stimulation Potentiates and Recruits Sensory-Evoked Cortical Population Activity
Abstract
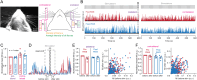
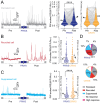
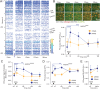
Sensory experience and learning are thought to be associated with plasticity of neocortical circuits. Repetitive sensory stimulation can induce long-term potentiation (LTP) of cortical excitatory synapses in anesthetized mice; however, it is unclear if these phenomena are associated with sustained changes in activity during wakefulness. Here we used time-lapse, calcium imaging of layer (L) 2/3 neurons in the primary somatosensory cortex (S1), in awake male mice, to assess the effects of a bout of rhythmic whisker stimulation (RWS) at a frequency by which rodents sample objects. We found that RWS induced a 1 h increase in whisker-evoked L2/3 neuronal activity in most cells. This was not observed for whiskers functionally connected to distant cortical columns. We also found that RWS could heterogeneously recruit or suppress whisker-evoked activity in different populations of neurons. Vasoactive intestinal-peptide-expressing (VIP) interneurons, which promote plasticity through disinhibition of pyramidal neurons, were found to exclusively elevate activity during RWS. These findings indicate that cortical neurons' representation of sensory input can be modulated over hours through repetitive sensory stimulation, which may be gated by activation of disinhibitory circuits.
Keywords: VIP interneurons; barrel cortex; disinhibition; long-term potentiation; somatosensory cortex; synaptic plasticity.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

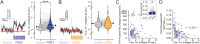

Similar articles
-
Peripuberty Is a Sensitive Period for Prefrontal Parvalbumin Interneuron Activity to Impact Adult Cognitive Flexibility.Dev Neurosci. 2025;47(2):127-138. doi: 10.1159/000539584. Epub 2024 Jun 3. Dev Neurosci. 2025. PMID: 38830346 Free PMC article.
-
Impairment in the homeostatic recruitment of layer 5/6 neurons following whisker stimulation in Fmr1 KO mice.Neurobiol Dis. 2025 Apr;207:106837. doi: 10.1016/j.nbd.2025.106837. Epub 2025 Feb 10. Neurobiol Dis. 2025. PMID: 39938578 Free PMC article.
-
Experience-Dependent Intrinsic Plasticity in Layer IV of Barrel Cortex at Whisking Onset.eNeuro. 2025 Aug 19;12(8):ENEURO.0252-25.2025. doi: 10.1523/ENEURO.0252-25.2025. Print 2025 Aug. eNeuro. 2025. PMID: 40730470 Free PMC article.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
-
Gonadotropin-releasing hormone (GnRH) analogues for premenstrual syndrome (PMS).Cochrane Database Syst Rev. 2025 Jun 10;6(6):CD011330. doi: 10.1002/14651858.CD011330.pub2. Cochrane Database Syst Rev. 2025. PMID: 40492482 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
