An explainable longitudinal multi-modal fusion model for predicting neoadjuvant therapy response in women with breast cancer
- PMID: 39511143
- PMCID: PMC11544255
- DOI: 10.1038/s41467-024-53450-8
An explainable longitudinal multi-modal fusion model for predicting neoadjuvant therapy response in women with breast cancer
Abstract
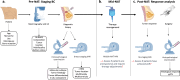
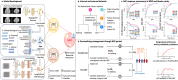
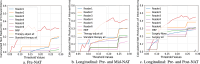
Multi-modal image analysis using deep learning (DL) lays the foundation for neoadjuvant treatment (NAT) response monitoring. However, existing methods prioritize extracting multi-modal features to enhance predictive performance, with limited consideration on real-world clinical applicability, particularly in longitudinal NAT scenarios with multi-modal data. Here, we propose the Multi-modal Response Prediction (MRP) system, designed to mimic real-world physician assessments of NAT responses in breast cancer. To enhance feasibility, MRP integrates cross-modal knowledge mining and temporal information embedding strategy to handle missing modalities and remain less affected by different NAT settings. We validated MRP through multi-center studies and multinational reader studies. MRP exhibited comparable robustness to breast radiologists, outperforming humans in predicting pathological complete response in the Pre-NAT phase (ΔAUROC 14% and 10% on in-house and external datasets, respectively). Furthermore, we assessed MRP's clinical utility impact on treatment decision-making. MRP may have profound implications for enrolment into NAT trials and determining surgery extensiveness.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA. Cancer J. Clin.73, 17–48 (2023). - PubMed
-
- Cortazar, P. & Geyer, C. E. Pathological complete response in neoadjuvant treatment of breast cancer. Ann. Surg. Oncol.22, 1441–1446 (2015). - PubMed
-
- van der Valk, M. J. et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the international watch & wait database (iwwd): an international multicentre registry study. Lancet391, 2537–2545 (2018). - PubMed
-
- Dattani, M. et al. Oncological and survival outcomes in watch and wait patients with a clinical complete response after neoadjuvant chemoradiotherapy for rectal cancer: a systematic review and pooled analysis. Ann. Surg.268, 955–967 (2018). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

