Back flux during anaerobic oxidation of butane support archaea-mediated alkanogenesis
- PMID: 39511174
- PMCID: PMC11543930
- DOI: 10.1038/s41467-024-53932-9
Back flux during anaerobic oxidation of butane support archaea-mediated alkanogenesis
Erratum in
-
Author Correction: Back flux during anaerobic oxidation of butane support archaea-mediated alkanogenesis.Nat Commun. 2025 Jan 7;16(1):453. doi: 10.1038/s41467-024-55458-6. Nat Commun. 2025. PMID: 39774673 Free PMC article. No abstract available.
Abstract
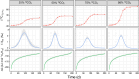
Microbial formation and oxidation of volatile alkanes in anoxic environments significantly impacts biogeochemical cycles on Earth. The discovery of archaea oxidizing volatile alkanes via deeply branching methyl-coenzyme M reductase variants, dubbed alkyl-CoM reductases (ACR), prompted the hypothesis of archaea-catalysed alkane formation in nature (alkanogenesis). A combination of metabolic modelling, anaerobic physiology assays, and isotope labeling of Candidatus Syntrophoarchaeum archaea catalyzing the anaerobic oxidation of butane (AOB) show a back flux of CO2 to butane, demonstrating reversibility of the entire AOB pathway. Back fluxes correlate with thermodynamics and kinetics of the archaeal catabolic system. AOB reversibility supports a biological formation of butane, and generally of higher volatile alkanes, helping to explain the presence of isotopically light alkanes and deeply branching ACR genes in sedimentary basins isolated from gas reservoirs.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Thauer, R. K., Kaster, A. K., Seedorf, H., Buckel, W. & Hedderich, R. Methanogenic archaea: ecologically relevant differences in energy conservation. Nat. Rev. Microbiol.6, 579–591 (2008). - PubMed
-
- Thauer, R. K. Anaerobic oxidation of methane with sulfate: on the reversibility of the reactions that are catalyzed by enzymes also involved in methanogenesis from CO2. Curr. Opin. Microbiol.14, 292–299 (2011). - PubMed
-
- Scheller, S., Goenrich, M., Boecher, R., Thauer, R. K. & Jaun, B. The key nickel enzyme of methanogenesis catalyses the anaerobic oxidation of methane. Nature465, 606–608 (2010). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- NNF22OC0071609/Novo Nordisk Fonden (Novo Nordisk Foundation)
- ERC-RA-0020/Helmholtz Association
- 101059607/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 Marie Skłodowska-Curie Actions (H2020 Excellent Science - Marie Skłodowska-Curie Actions)
- 12471341/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources

