Preoperative methionine restriction induces perivascular adipose tissue browning and improves vein graft remodeling in male mice
- PMID: 39511181
- PMCID: PMC11544300
- DOI: 10.1038/s41467-024-53844-8
Preoperative methionine restriction induces perivascular adipose tissue browning and improves vein graft remodeling in male mice
Abstract
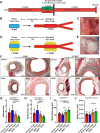
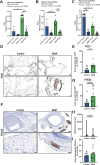
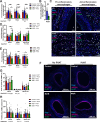
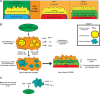
Short-term preoperative methionine restriction (MetR) is a promising translatable strategy to mitigate surgical injury response. However, its application to improve post-interventional vascular remodeling remains underexplored. Here we find that MetR protects from arterial intimal hyperplasia in a focal stenosis model and pathologic vascular remodeling following vein graft surgery in male mice. RNA sequencing reveals that MetR enhances browning in arterial (thoracic aorta) perivascular adipose tissue (PVAT) and induces it in venous (caval vein) PVAT. Specifically, Ppara is highly upregulated in PVAT-adipocytes upon MetR. Furthermore, MetR dampens the postoperative pro-inflammatory response to surgery in PVAT-macrophages in vivo and in vitro. This study shows that the detrimental effects of dysfunctional PVAT on vascular remodeling can be reversed by MetR, and identifies pathways involved in MetR-induced browning of PVAT. Furthermore, we demonstrate the potential of short-term preoperative MetR as a simple intervention to ameliorate vascular remodeling after vascular surgery.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Update of
-
Short-term Pre-operative Methionine Restriction Induces Browning of Perivascular Adipose Tissue and Improves Vein Graft Remodeling in Mice.bioRxiv [Preprint]. 2023 Nov 4:2023.11.02.565269. doi: 10.1101/2023.11.02.565269. bioRxiv. 2023. Update in: Nat Commun. 2024 Nov 7;15(1):9652. doi: 10.1038/s41467-024-53844-8. PMID: 37961405 Free PMC article. Updated. Preprint.
References
-
- Farber, A. et al. Surgery or endovascular therapy for chronic limb-threatening ischemia. N. Engl. J. Med.387, 2305–2316 (2022). - PubMed
-
- Yahagi, K. et al. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat. Rev. Cardiol.13, 79–98 (2016). - PubMed
-
- de Vries, M. R., Simons, K. H., Jukema, J. W., Braun, J. & Quax, P. H. A. Vein graft failure: from pathophysiology to clinical outcomes. Nat. Rev. Cardiol.13, 451–470 (2016). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

