mRNA delivery enabled by metal-organic nanoparticles
- PMID: 39511206
- PMCID: PMC11544223
- DOI: 10.1038/s41467-024-53969-w
mRNA delivery enabled by metal-organic nanoparticles
Abstract
mRNA therapeutics are set to revolutionize disease prevention and treatment, inspiring the development of platforms for safe and effective mRNA delivery. However, current mRNA delivery platforms face some challenges, including limited organ tropism for nonvaccine applications and inflammation induced by cationic nanoparticle components. Herein, we address these challenges through a versatile, noncationic nanoparticle platform whereby mRNA is assembled into a poly(ethylene glycol)-polyphenol network stabilized by metal ions. Screening a range of components and relative compositional ratios affords a library of stable, noncationic, and highly biocompatible metal-organic nanoparticles with robust mRNA transfection in vitro and in mice. Intravenous administration of the lead mRNA-containing metal-organic nanoparticles enables predominant protein expression and gene editing in the brain, liver, and kidney, while organ tropism is tuned by varying nanoparticle composition. This study opens an avenue for realizing metal-organic nanoparticle-enabled mRNA delivery, offering a modular approach to assembling mRNA therapeutics for health applications.
© 2024. The Author(s).
Conflict of interest statement
F.C., J.C., Y.G., and C.C.-J. have filed a patent application for this technology. F.C. is a shareholder of Messenger Bio Pty Ltd. The remaining authors declare no competing interests.
Figures


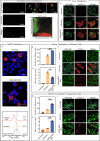
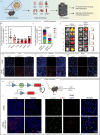
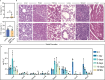

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

