Distinct pathway of multiferroic silver-decorated zinc ferrite nanocatalyst performance for Acinate insecticide oxidation
- PMID: 39511358
- PMCID: PMC11543926
- DOI: 10.1038/s41598-024-78678-8
Distinct pathway of multiferroic silver-decorated zinc ferrite nanocatalyst performance for Acinate insecticide oxidation
Abstract
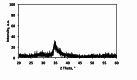
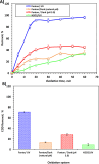
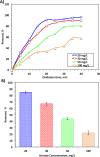
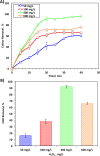
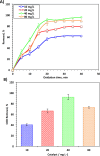
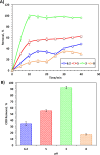
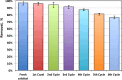
The current study investigating the preparation and application of a Multiferroic nano-scale silver zinc ferrite substance (Ag0.5Zn0.5Fe2O4 nanocatalyst) has been established. Multiferroic silver zinc ferrite substance is prepared by co-precipitation technique as hybridized composite. This synethsized nanoparticles was characterized via X-ray diffraction (XRD), high-resolution transmission electron microscopy (HRTEM) as well as Scanning Electron Miscospopy (SEM). Such nanoparticles are used as a sustainable recyclable photo-Fenton's reagent precursor for treating insecticide in wastewater. The results revealed a high Acinate oxidation rate reached to 97% removal within 40 min of irradiance time. To increase the performance, the operating variables are optimized. pH 3.0 and 40 and 400 mg/L for Ag0.5Zn0.5Fe2O4 and hydrogen peroxide, respectively are identified as the optimum values. Also, kinetics and thermodynamic are evaluated and the reaction is subsequent the first-order kinetics model and exothermic and non-spontaneous in nature with a low energy barrier of 35.02 kJ/mol. The advantage of Ag0.5Zn0.5Fe2O4 catalyst is its sustainability since it recovered for multiple reuse with a high activity reached to 80% removal rate after six cyclic use compared to 97% of fresh catalyst use.
Keywords: Fenton reaction; Insecticide; Nanomaterial; Oxidation; Photocatalyst; Wastewater.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Abdollahzadeh, H. et al. Efficiency of activated carbon prepared from scrap tires magnetized by Fe3O4 nanoparticles: Characterisation and its application for removal of reactive blue19 from aquatic solutions. Int. J. Environ. Anal. Chem.10.1080/03067319.2020.1745199 (2020).
-
- Ahmadi, M., Behin, J. & Mahnam, A. R. Kinetics and thermodynamics of peroxydisulfate oxidation of reactive yellow 84. J. Saudi Chem. Soci20, 644–650 (2016).
-
- Al Momani, F., Shawaqfah, M., Shawaqfeh, A. & Al-Shannag, M. Impact of Fenton and ozone on oxidation of wastewater containing nitroaromatic compounds. J. Environ. Sci.20, 675 (2008). - PubMed
-
- Argun, M. E. & Karatas, M. Application of Fenton process for decolorization of reactive black 5 from synthetic wastewater: Kinetics and thermodynamics. Prog. Sust. Energy30: 540 (2011).
-
- Abdou, K. A., Mohammed, A. N., Moselhy, W. & Farghali, A. A. Assessment of modified rice husk and sawdust as bio-adsorbent for heavy metals removal using nano particles in fish farm. Asian J. Anim. Vet. Adv.13, 180–188 (2018).
Grants and funding
LinkOut - more resources
Full Text Sources

