Automated decision making in Barrett's oesophagus: development and deployment of a natural language processing tool
- PMID: 39511374
- PMCID: PMC11544096
- DOI: 10.1038/s41746-024-01302-6
Automated decision making in Barrett's oesophagus: development and deployment of a natural language processing tool
Abstract
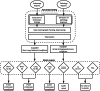
Manual decisions regarding the timing of surveillance endoscopy for premalignant Barrett's oesophagus (BO) is error-prone. This leads to inefficient resource usage and safety risks. To automate decision-making, we fine-tuned Bidirectional Encoder Representations from Transformers (BERT) models to categorize BO length (EndoBERT) and worst histopathological grade (PathBERT) on 4,831 endoscopy and 4,581 pathology reports from Guy's and St Thomas' Hospital (GSTT). The accuracies for EndoBERT test sets from GSTT, King's College Hospital (KCH), and Sandwell and West Birmingham Hospitals (SWB) were 0.95, 0.86, and 0.99, respectively. Average accuracies for PathBERT were 0.93, 0.91, and 0.92, respectively. A retrospective analysis of 1640 GSTT reports revealed a 27% discrepancy between endoscopists' decisions and model recommendations. This study underscores the development and deployment of NLP-based software in BO surveillance, demonstrating high performance at multiple sites. The analysis emphasizes the potential efficiency of automation in enhancing precision and guideline adherence in clinical decision-making.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Robertson, C. S., Mayberry, J. F., Nicholson, D. A., James, P. D. & Atkinson, M. Value of endoscopic surveillance in the detection of neoplastic change in Barrett’s oesophagus. Br. J. Surg.75, 760–763 (2005). - PubMed
-
- Hameeteman, W., Tytgat, G. N. J., Houthoff, H. J. & van den Tweel, J. G. Barrett’s Esophagus; Development of Dysplasia and Adenocarcinoma. Gastroenterology96, 1249–1256 (1989). - PubMed
-
- Fitzgerald, R. C. et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut.63, 7–42 (2014). - PubMed
LinkOut - more resources
Full Text Sources

