Metabolic profiling unveils enhanced antibacterial synergy of polymyxin B and teixobactin against multi-drug resistant Acinetobacter baumannii
- PMID: 39511424
- PMCID: PMC11543821
- DOI: 10.1038/s41598-024-78769-6
Metabolic profiling unveils enhanced antibacterial synergy of polymyxin B and teixobactin against multi-drug resistant Acinetobacter baumannii
Abstract
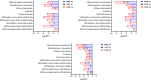
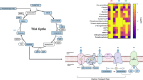
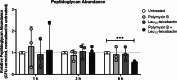
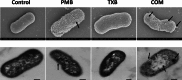
This untargeted metabolomics study investigated the synergistic antibacterial activity of polymyxin B and Leu10-teixobactin, a depsipeptide inhibitor of cell wall biosynthesis. Checkerboard microdilution assays revealed a significant synergy against polymyxin-susceptible and -resistant A. baumannii, excluding lipopolysaccharide-deficient variants. Time-kill assays confirmed bactericidal synergy, reducing bacterial burden by approximately 4-6-log10CFU/mL. The combination (2xMIC polymyxin B and 0.5xMIC Leu10-teixobactin) prevented bacterial regrowth after 24 h, indicating sustained efficacy against the emergence of resistant mutants. The analysis of A. baumannii ATCC™ 19606 metabolome demonstrated that the polymyxin B-Leu10-teixobactin combination produced more pronounced perturbation compared to the individual antibiotics across all time points (1, 3 and 6 h). Pathway analysis revealed that lipid metabolism, cell envelope biogenesis, and cellular respiration were predominantly impacted by the combination, and to a lesser extent by polymyxin B monotherapy. Leu10-teixobactin treatment alone had only a minor impact on the metabolome, primarily at the 6 h time point. Peptidoglycan assays confirmed the combination's concerted deleterious effects on bacterial cell envelope integrity. Electron microscopy further substantiated these findings, revealing pronounced cell envelope damage, membrane blebbing, and vacuole formation. These findings highlight the potential of the polymyxin B-Leu10-teixobactin combination as an effective treatment in preventing resistance in A. baumannii.
Keywords: A. baumannii; Antimicrobial resistance; Metabolomics; Polymyxin B; Teixobactin.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Russo, A. et al. Bloodstream infections caused by carbapenem-resistant Acinetobacter baumannii: Clinical features, therapy and outcome from a multicenter study. J. Infect.79 (2), 130–138 (2019). - PubMed
-
- Tacconelli, E. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development. (2017).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

