The inflammasome-activating poxvirus peptide IAMP29 promotes antimicrobial and anticancer responses
- PMID: 39511430
- PMCID: PMC11612179
- DOI: 10.1038/s12276-024-01339-3
The inflammasome-activating poxvirus peptide IAMP29 promotes antimicrobial and anticancer responses
Abstract
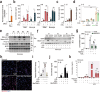
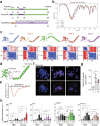
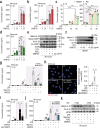
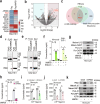
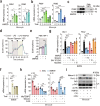
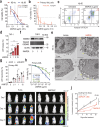
Poxviruses are implicated in a variety of infectious diseases; however, little is known about the molecular mechanisms that underlie the immune response during poxvirus infection. We investigated the function and mechanisms of the monkeypox virus envelope protein (A30L) and its core peptide (IAMP29) during the activation of innate immune responses. The A30L protein and its core peptide, IAMP29 (a 29-amino-acid inflammasome-activating peptide encompassing His40 to Asp69 of A30L), strongly activated the nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain-containing 3 (NLRP3) inflammasome by inducing the production of mitochondrial reactive oxygen species in human monocytes. Specifically, IAMP29 triggered metabolic reprogramming toward glycolysis and interacted with pyruvate kinase M isoforms (PKM1 and PKM2), thus activating the NLRP3 inflammasome and interleukin (IL)-1β production in human monocytes and murine macrophages. In human primary monocyte-derived macrophages, IAMP29-induced inflammasome activation promoted an antimicrobial response to rapidly growing non-tuberculous mycobacteria. Furthermore, IAMP29 exhibited cytotoxic activity against leukemia cells, which was mediated by pyroptosis and apoptosis. These findings provide insights into the immunological function of the poxvirus envelope peptide and suggest its therapeutic potential.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

