Non-fermented and fermented milk intake in relation to risk of ischemic heart disease and to circulating cardiometabolic proteins in swedish women and men: Two prospective longitudinal cohort studies with 100,775 participants
- PMID: 39511582
- PMCID: PMC11546556
- DOI: 10.1186/s12916-024-03651-1
Non-fermented and fermented milk intake in relation to risk of ischemic heart disease and to circulating cardiometabolic proteins in swedish women and men: Two prospective longitudinal cohort studies with 100,775 participants
Abstract
Background: The effect of milk on the risk of ischemic heart disease (IHD) and acute myocardial infarction (MI) is unclear. We aimed to examine the association between non-fermented and fermented milk consumption on these endpoints and investigate the relationship between milk intake and cardiometabolic-related proteins in plasma.
Methods: Our study is based on two Swedish prospective cohort studies that included 59,998 women and 40,777 men without IHD or cancer at baseline who provided repeated measures of diet and lifestyle factors and plasma proteomics data in two subcohorts. Through registry linkage, 17,896 cases with IHD were documented during up to 33 years of follow-up, including 10,714 with MI. We used time-updated multivariable Cox regression analysis to examine non-fermented or fermented milk intake with time to IHD or MI. Using high-throughput multiplex immunoassays, 276 cardiometabolic plasma proteins were measured in two subcohorts. We applied multivariable-adjusted regression models using a discovery-replication design to examine protein associations with increasing consumption of non-fermented or fermented milk.
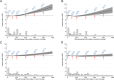
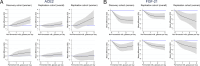
Results: The results for non-fermented milk differed by sex (p-value for interaction = 0.01). In women, we found a pattern of successively greater risk of IHD and MI at non-fermented milk intake levels higher than 1.5 glasses/day. Compared with an intake of 0.5 glass/day (100 mL/day), non-fermented milk intake of 2 glasses/day in women conferred a multivariable-adjusted hazard ratio (HR) of 1.05 (95% CI 1.01-1.08) for IHD, an intake of 3 glasses/day an HR of 1.12 (95% CI 1.06-1.19), and an intake of 4 glasses/day an HR of 1.21 (95% CI 1.10-1.32). Findings were similar for whole, medium-fat, and low-fat milk. We did not detect higher risks of IHD with increasing milk intakes in men. Fermented milk intake was unrelated to the risk of IHD or MI in either sex. Increasing non-fermented milk intake in women was robustly associated with a higher concentration of plasma ACE2 and a lower concentration of FGF21.
Conclusions: We show a positive association between high amounts of non-fermented milk intake and IHD in women but not men. We suggest metabolic pathways related to ACE2 and FGF21 potentially underlie the association.
Keywords: ACE2; Cohort; FGF21; Fermented; Ischemic heart disease; Milk; Myocardial infarction; Non-fermented.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;37(42):3232–45. 10.1093/eurheartj/ehw334. - PubMed
-
- Lichtenstein AH, Appel LJ, Vadiveloo M, Hu FB, Kris-Etherton PM, Rebholz CM, et al. 2021 dietary guidance to improve cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2021;144(23):e472–87. 10.1161/CIR.0000000000001031. - PubMed
-
- Dehghan M, Mente A, Rangarajan S, Sheridan P, Mohan V, Iqbal R, et al. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): a prospective cohort study. Lancet. 2018;392(10161):2288–97. 10.1016/S0140-6736(18)31812-9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

