Soluble and EV-bound CD27 act as antagonistic biomarkers in patients with solid tumors undergoing immunotherapy
- PMID: 39511626
- PMCID: PMC11545160
- DOI: 10.1186/s13046-024-03215-4
Soluble and EV-bound CD27 act as antagonistic biomarkers in patients with solid tumors undergoing immunotherapy
Abstract
Background: The major breakthrough in cancer therapy with immune checkpoint inhibitors (ICIs) has highlighted the important role of immune checkpoints in antitumoral immunity. However, most patients do not achieve durable responses, making biomarker research in this setting essential. CD27 is a well known costimulatory molecule, however the impact of its soluble form in ICI is poorly investigated. Therefore, we aimed at testing circulating concentrations of soluble CD27 (sCD27) and CD27 bound to extracellular vesicles (EVs) as potential biomarkers to predict response and overall survival (OS) in patients undergoing ICI.
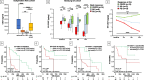
Methods: Serum and plasma levels of sCD27 were assessed by immunoassay in three patient cohorts (n = 187) with advanced solid malignancies including longitudinal samples (n = 126): a training (n = 84, 210 specimens, Aachen ICI) and validation cohort (n = 70, 70 specimens, Hamburg ICI), both treated with ICI therapy, and a second independent validation cohort (n = 33, 33 specimens, Hamburg non-ICI) undergoing systemic therapy without any ICI. In a subset (n = 36, 36 baseline and 108 longitudinal specimens), EV-bound CD27 from serum was measured, while EV characterization studies were conducted on a fourth cohort (n = 45).
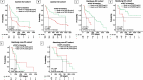
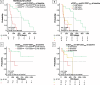
Results: In the Aachen and Hamburg ICI cohorts, patients with lower circulating sCD27 levels before and during ICI therapy had a significantly longer progression-free survival (PFS) and OS compared to patients with higher levels, a finding that was confirmed by multivariate analysis (MVA) (Aachen ICI: pPFS = 0.012, pOS = 0.001; Hamburg ICI: pPFS = 0.040, pOS = 0.004) and after randomly splitting both cohorts into training and validation. This phenomenon was not observed in the Hamburg non-ICI cohort, providing a rationale for the predictive biomarker role of sCD27 in immune checkpoint blockade. Remarkably, EV-bound CD27 baseline levels and dynamics during ICI therapy also emerged as potent predictive biomarkers, acting however antagonistically to soluble sCD27, i.e. higher levels were associated with PFS and OS benefit. Combining both molecules ("multi-CD27" score) enhanced the predictive ability (HRPFS: 17.21 with p < 0.001, HROS: 6.47 with p = 0.011).
Conclusion: Soluble and EV-bound CD27 appear to have opposing immunomodulatory functions and may represent easily measurable, non-invasive prognostic markers to predict response and survival in patients undergoing ICI therapy.
Keywords: Biomarker; Immunotherapy; Liquid biopsy; PD-1; Prognosis; Soluble immune checkpoints.
© 2024. The Author(s).
Conflict of interest statement
JvF has received honoraria from Roche and AstraZeneca. Further authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

