Nanobubble Contrast Enhanced Ultrasound Imaging: A Review
- PMID: 39511794
- PMCID: PMC11567054
- DOI: 10.1002/wnan.2007
Nanobubble Contrast Enhanced Ultrasound Imaging: A Review
Abstract
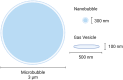
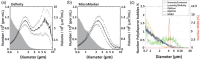
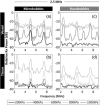
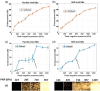
Contrast-enhanced ultrasound is currently used worldwide with clinical indications in cardiology and radiology, and it continues to evolve and develop through innovative technological advancements. Clinically utilized contrast agents for ultrasound consist of hydrophobic gas microbubbles stabilized with a biocompatible shell. These agents are used commonly in echocardiography, with emerging applications in cancer diagnosis and therapy. Microbubbles are a blood pool agent with diameters between 1 and 10 μm, which precludes their use in other extravascular applications. To expand the potential use of contrast-enhanced ultrasound beyond intravascular applications, sub-micron agents, often called nanobubbles or ultra-fine bubbles, have recently emerged as a promising tool. Combining the principles of ultrasound imaging with the unique properties of nanobubbles (high concentration and small size), recent work has established their imaging potential. Contrast-enhanced ultrasound imaging using these agents continues to gain traction, with new studies establishing novel imaging applications. We highlight the recent achievements in nonlinear nanobubble contrast imaging, including a discussion on nanobubble formulations and their acoustic characteristics. Ultrasound imaging with nanobubbles is still in its early stages, but it has shown great potential in preclinical research and animal studies. We highlight unexplored areas of research where the capabilities of nanobubbles may offer new advantages. As technology advances, this technique may find applications in various areas of medicine, including cancer detection and treatment, cardiovascular imaging, and drug delivery.
Keywords: contrast; microbubbles; nanobubbles; nonlinear; ultrasound.
© 2024 The Author(s). WIREs Nanomedicine and Nanobiotechnology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Abenojar, E. C. , Nittayacharn P., de Leon A. C., et al. 2019. “Effect of Bubble Concentration on the In Vitro and In Vivo Performance of Highly Stable Lipid Shell‐Stabilized Micro‐ and Nanoscale Ultrasound Contrast Agents.” Langmuir 35, no. 31: 10192–10202. 10.1021/acs.langmuir.9b00462. - DOI - PubMed
-
- Alheshibri, M. , and Craig V. S. J.. 2018. “Differentiating Between Nanoparticles and Nanobubbles by Evaluation of the Compressibility and Density of Nanoparticles.” Journal of Physical Chemistry C 122, no. 38: 21998–22007. 10.1021/acs.jpcc.8b07174. - DOI
-
- Aliabouzar, M. , Kripfgans O. D., Brian Fowlkes J., and Fabiilli M. L.. 2023. “Bubble Nucleation and Dynamics in Acoustic Droplet Vaporization: A Review of Concepts, Applications, and New Directions.” Zeitschrift für Medizinische Physik 33, no. 3: 387–406. 10.1016/j.zemedi.2023.01.004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

