Association of amyloid and cardiovascular risk with cognition: Findings from KBASE
- PMID: 39511852
- PMCID: PMC11667546
- DOI: 10.1002/alz.14290
Association of amyloid and cardiovascular risk with cognition: Findings from KBASE
Abstract
Background: Limited research has explored the effect of cardiovascular risk and amyloid interplay on cognitive decline in East Asians.
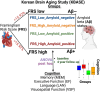
Methods: Vascular burden was quantified using Framingham's General Cardiovascular Risk Score (FRS) in 526 Korean Brain Aging Study (KBASE) participants. Cognitive differences in groups stratified by FRS and amyloid positivity were assessed at baseline and longitudinally.
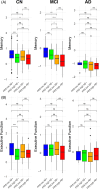
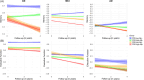
Results: Baseline analyses revealed that amyloid-negative (Aβ-) cognitively normal (CN) individuals with high FRS had lower cognition compared to Aβ- CN individuals with low FRS (p < 0.0001). Longitudinally, amyloid pathology predominantly drove cognitive decline, while FRS alone had negligible effects on cognition in CN and mild cognitive impairment (MCI) groups.
Conclusion: Our findings indicate that managing vascular risk may be crucial in preserving cognition in Aβ- individuals early on and before the clinical manifestation of dementia. Within the CN and MCI groups, irrespective of FRS status, amyloid-positive individuals had worse cognitive performance than Aβ- individuals.
Highlights: Vascular risk significantly affects cognition in amyloid-negative older Koreans. Amyloid-negative CN older adults with high vascular risk had lower baseline cognition. Amyloid pathology drives cognitive decline in CN and MCI, regardless of vascular risk. The study underscores the impact of vascular health on the AD disease spectrum.
Keywords: Alzheimer's disease; CN; Framingham Risk Score; Korean older adults; MCI; amyloid; cognition; longitudinal; vascular risk factors.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Andrew J. Saykin has received support from Avid Radiopharmaceuticals, a subsidiary of Eli Lilly (in‐kind contribution of PET tracer precursor), and participated in Scientific Advisory Boards (Bayer Oncology, Eisai, Novo Nordisk, and Siemens Medical Solutions USA, Inc) and an Observational Study Monitoring Board (MESA, NIH NHLBI), as well as several other NIA External Advisory Committees. He also serves as Editor‐in‐Chief of
Figures
References
MeSH terms
Substances
Grants and funding
- T32 AG071444/AG/NIA NIH HHS/United States
- R01 AG019771/AG/NIA NIH HHS/United States
- R01 LM013463/GF/NIH HHS/United States
- HI18C0630/Korean government
- R01 AG019771/GF/NIH HHS/United States
- U01 AG072177/GF/NIH HHS/United States
- U19 AG024904/AG/NIA NIH HHS/United States
- R01 AG068193/GF/NIH HHS/United States
- RS-2023-KH136195/Korean government
- RS-2022-00165636/Korean government
- R01 LM013463/LM/NLM NIH HHS/United States
- New Faculty Startup Fund, Seoul National University
- U01 AG072177/AG/NIA NIH HHS/United States
- P30 AG010133/AG/NIA NIH HHS/United States
- U01AG072177/ADNI Health Equity Scholarship Program, subaward of NIA
- R37 AG057739/AG/NIA NIH HHS/United States
- U19 AG074879/AG/NIA NIH HHS/United States
- NRF-2014M3C7A1046042/Korean government
- P30 AG072976/GF/NIH HHS/United States
- T32 AG071444/GF/NIH HHS/United States
- U01 AG068057/AG/NIA NIH HHS/United States
- P30 AG010133/GF/NIH HHS/United States
- U01 AG068057/GF/NIH HHS/United States
- R01 AG057739/GF/NIH HHS/United States
- U19 AG024904/GF/NIH HHS/United States
- U19 AG074879/GF/NIH HHS/United States
- R01 AG057739/AG/NIA NIH HHS/United States
- R01 AG068193/AG/NIA NIH HHS/United States
- R01 LM012535/LM/NLM NIH HHS/United States
- P30 AG072976/AG/NIA NIH HHS/United States
- HI19C0149/Korean government
LinkOut - more resources
Full Text Sources
Medical

