Enhancement of Glutamate Uptake as Novel Antiseizure Approach: Preclinical Proof of Concept
- PMID: 39512205
- PMCID: PMC11740271
- DOI: 10.1002/ana.27124
Enhancement of Glutamate Uptake as Novel Antiseizure Approach: Preclinical Proof of Concept
Abstract
Objective: Excitotoxicity is a common hallmark of epilepsy and other neurological diseases associated with elevated extracellular glutamate levels. Thus, here, we studied the protective effects of (R)-AS-1, a positive allosteric modulator (PAM) of glutamate uptake in epilepsy models.
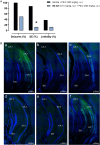
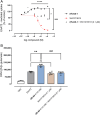
Methods: (R)-AS-1 was evaluated in a range of acute and chronic seizure models, while its adverse effect profile was assessed in a panel of standard tests in rodents. The effect of (R)-AS-1 on glutamate uptake was assessed in COS-7 cells expressing the transporter. WAY 213613, a selective competitive EAAT2 inhibitor, was used to probe the reversal of the enhanced glutamate uptake in the same transporter expression system. Confocal microscopy and Western blotting analyses were used to study a potential influence of (R)-AS-1 on GLT-1 expression in mice.
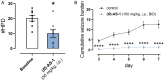
Results: (R)-AS-1 showed robust protection in a panel of animal models of seizures and epilepsy, including the maximal electroshock- and 6 Hz-induced seizures, corneal kindling, mesial temporal lobe epilepsy, lamotrigine-resistant amygdala kindling, as well as seizures induced by pilocarpine or Theiler's murine encephalomyelitis virus. Importantly, (R)-AS-1 displayed a favorable adverse effect profile in the rotarod, the minimal motor impairment, and the Irwin tests. (R)-AS-1 enhanced glutamate uptake in vitro and this effect was abolished by WAY 213613, while no influence on GLT-1 expression in vivo was observed after repeated treatment.
Interpretation: Collectively, our results show that (R)-AS-1 has favorable tolerability and provides robust preclinical efficacy against seizures. Thus, allosteric enhancement of EAAT2 function could offer a novel therapeutic strategy for treatment of epilepsy and potentially other neurological disorders associated with glutamate excitotoxicity. ANN NEUROL 2025;97:344-357.
© 2024 The Author(s). Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Prof. Krzysztof Kamiński is the CSO in iQure Pharma, Princeton, NJ, US. The company is developing
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

