Circulating mucosal-like IgA responses increase with severity of Puumala orthohantavirus-caused hemorrhagic fever with renal syndrome
- PMID: 39512345
- PMCID: PMC11540702
- DOI: 10.3389/fimmu.2024.1480041
Circulating mucosal-like IgA responses increase with severity of Puumala orthohantavirus-caused hemorrhagic fever with renal syndrome
Abstract
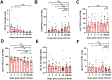
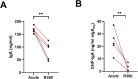
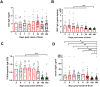
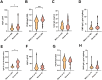
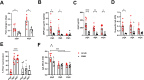
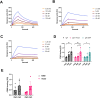
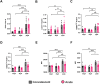
Old World Orthohantaviruses cause hemorrhagic fever with renal syndrome (HFRS) characterized by increased vascular permeability and acute kidney injury (AKI). Despite the systemic nature of the disease, the virus enters humans through inhalation and therefore initially encounters the immunoglobulin class A (IgA) dominated mucosal immune system. Herein, we characterized systemic IgA responses and their potential relationship to the mucosal immune activation by examining blood samples obtained from patients hospitalized due to acute Puumala orthohantavirus infection. Our findings reveal increased frequencies of putative IgA-expressing circulating mucosal-associated B1 cells and plasmablasts, as well as elevated levels of polyreactive, polymeric, virus-specific and secretory IgA in the acute stage of the disease. Importantly, the levels of circulating virus-specific and secretory IgA, as well as the putative IgA+ B1 cells, increased with the severity of AKI. Furthermore, circulating polymeric IgA displayed enhanced effector functions by forming stable complexes with the IgA receptor CD89 and induced pro-inflammatory neutrophil responses. These results suggest that excessive levels of circulating mucosal-like IgA might serve as a biomarker for HFRS disease progression.
Keywords: HFRS; IgA; hantavirus; mucosal immunity; neutrophils.
Copyright © 2024 Cabrera, Buckner, Then, Mäki, Vapalahti, Vaheri, Hepojoki, Tietäväinen, Mäkelä, Mustonen and Strandin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Viral load and humoral immune response in association with disease severity in Puumala hantavirus-infected patients--implications for treatment.Clin Microbiol Infect. 2014 Mar;20(3):235-41. doi: 10.1111/1469-0691.12259. Epub 2013 Jun 7. Clin Microbiol Infect. 2014. PMID: 23742660 Free PMC article.
-
MAIT cell activation is associated with disease severity markers in acute hantavirus infection.Cell Rep Med. 2021 Mar 16;2(3):100220. doi: 10.1016/j.xcrm.2021.100220. eCollection 2021 Mar 16. Cell Rep Med. 2021. PMID: 33763658 Free PMC article.
-
Hantavirus-specific IgA in saliva and viral antigen in the parotid gland in patients with hemorrhagic fever with renal syndrome.J Med Virol. 2011 May;83(5):864-70. doi: 10.1002/jmv.22040. Epub 2011 Feb 25. J Med Virol. 2011. PMID: 21360546
-
Severity Biomarkers in Puumala Hantavirus Infection.Viruses. 2021 Dec 28;14(1):45. doi: 10.3390/v14010045. Viruses. 2021. PMID: 35062248 Free PMC article. Review.
-
Innate and adaptive immune responses against human Puumala virus infection: immunopathogenesis and suggestions for novel treatment strategies for severe hantavirus-associated syndromes.J Intern Med. 2019 May;285(5):510-523. doi: 10.1111/joim.12876. Epub 2019 Feb 17. J Intern Med. 2019. PMID: 30663801 Free PMC article. Review.
Cited by
-
Analysis of Processing Impact on Raspberries Based on Broad-Spectrum Metabolomics.Metabolites. 2025 Jun 26;15(7):435. doi: 10.3390/metabo15070435. Metabolites. 2025. PMID: 40710535 Free PMC article.
References
-
- Klingström J, Smed-Sörensen A, Maleki KT, Solà-Riera C, Ahlm C, Björkström NK, et al. . Innate and adaptive immune responses against human Puumala virus infection: immunopathogenesis and suggestions for novel treatment strategies for severe hantavirus-associated syndromes. J Intern Med. (2019) 285:510–23. doi: 10.1111/joim.12876 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

