Vascular and nerve biomarkers in thigh skin biopsies differentiate painful from painless diabetic peripheral neuropathy
- PMID: 39512388
- PMCID: PMC11543357
- DOI: 10.3389/fpain.2024.1485420
Vascular and nerve biomarkers in thigh skin biopsies differentiate painful from painless diabetic peripheral neuropathy
Abstract
Background: Identifying distinct mechanisms and biomarkers for painful diabetic peripheral neuropathy (DPN) is required for advancing the treatment of this major global unmet clinical need. We previously provided evidence in calf skin biopsies that disproportion between reduced sensory small nerve fibers and increased blood vessels may distinguish painful from non-painful DPN. We proposed that overexposure of the reduced nerve fibers in DPN to increased hypoxemia-induced vasculature and related algogenic factors, e.g., nerve growth factor (NGF), leads to neuropathic pain. To further investigate this proposed mechanism, we have now studied more proximal thigh skin biopsies, to see if the same disproportion between increased vasculature and decreased nerve fibers generally differentiates painful DPN from painless DPN.
Methods: A total of 28 subjects with type 2 diabetes (T2DM) and 13 healthy volunteers (HV) underwent detailed clinical and neurophysiological assessments, based on the neuropathy composite score of the lower limbs [NIS(LL)] plus 7 tests. T2DM subjects were subsequently divided into three groups: painful DPN (n = 15), painless DPN (n = 7), and no DPN (n = 6). All subjects underwent skin punch biopsy from the upper lateral thigh 20 cm below the anterior iliac spine.
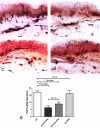
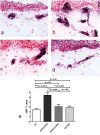
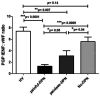
Results: Skin biopsies showed decreased PGP 9.5-positive intraepidermal nerve fiber (IENF) density in both painful DPN (p < 0.0001) and painless DPN (p = 0.001). Vascular marker von Willebrand Factor (vWF) density was markedly increased in painful DPN vs. other groups, including painless DPN (p = 0.01). There was a resulting significant decrease in the ratio of intraepidermal nerve fiber density to vasculature and PGP9.5 to vWF, in painful DPN vs. painless DPN (p = 0.05). These results were similar in pattern to those observed in these HV and T2DM groups previously in distal calf biopsies; however, the increase in vWF was much higher and nerve fiber density much lower in the calf than thigh for painful DPN. Thigh skin vWF density was significantly correlated with several metabolic (waist/hip ratio, HbA1c), clinical (e.g., pain score), and neurophysiological measures.
Conclusion: This study supports our proposal that increased dermal vasculature, and its disproportionate ratio to reduced nociceptors, may help differentiate painful DPN from painless DPN. This disproportion is greater in the distal calf than the proximal thigh skin; hence, neuropathic pain in DPN is length-dependent and first localized to the distal lower limbs, mainly feet.
Keywords: IENF; biomarkers; pain; painful diabetic neuropathy; skin; type 2 diabetes; vascular; von Willebrand factor.
© 2024 Sloan, Donatien, Privitera, Shillo, Caunt, Selvarajah, Anand and Tesfaye.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

