Phage-encoded depolymerases as a strategy for combating multidrug-resistant Acinetobacter baumannii
- PMID: 39512587
- PMCID: PMC11540826
- DOI: 10.3389/fcimb.2024.1462620
Phage-encoded depolymerases as a strategy for combating multidrug-resistant Acinetobacter baumannii
Abstract
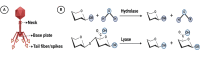
Acinetobacter baumannii, a predominant nosocomial pathogen, represents a grave threat to public health due to its multiple antimicrobial resistance. Managing patients afflicted with severe infections caused by multiple drug-resistant A. baumannii is particularly challenging, given the associated high mortality rates and unfavorable prognoses. The diminishing efficacy of antibiotics against this superbug underscores the urgent necessity for novel treatments or strategies to address this formidable issue. Bacteriophage-derived polysaccharide depolymerase enzymes present a potential approach to combating this pathogen. These enzymes target and degrade the bacterial cell's exopolysaccharide, capsular polysaccharide, and lipopolysaccharide, thereby disrupting biofilm formation and impairing the bacteria's defense mechanisms. Nonetheless, the narrow host range of phage depolymerases limits their therapeutic efficacy. Despite the benefits of these enzymes, phage-resistant strains have been identified, highlighting the complexity of phage-host interactions and the need for further investigation. While preliminary findings are encouraging, current investigations are limited, and clinical trials are imperative to advance this treatment approach for broader clinical applications. This review explores the potential of phage-derived depolymerase enzymes against A. baumannii infections.
Keywords: bacteriophage; biofilm; depolymerase; drug resistance; polysaccharide.
Copyright © 2024 Islam, Mahbub, Shin and Oh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Al-Shamiri M. M., Zhang S., Mi P., Liu Y., Xun M., Yang E., et al. . (2021). Phenotypic and genotypic characteristics of Acinetobacter baumannii enrolled in the relationship among antibiotic resistance, biofilm formation and motility. Microb. Pathogen. 155, 104922. doi: 10.1016/j.micpath.2021.104922 - DOI - PubMed
-
- Anyaegbunam N. J., Anekpo C. C., Anyaegbunam Z. K. G., Doowuese Y., Chinaka C. B., Odo O. J., et al. . (2022). The resurgence of phage-based therapy in the era of increasing antibiotic resistance: From research progress to challenges and prospects. Microbiol. Res. 264, 127155. doi: 10.1016/j.micres.2022.127155 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

