Extended conformations of bifurcating electron transfer flavoprotein constitute up to half the population, possibly mediating conformational change
- PMID: 39512923
- PMCID: PMC11536132
- DOI: 10.1039/d4sc04544k
Extended conformations of bifurcating electron transfer flavoprotein constitute up to half the population, possibly mediating conformational change
Abstract
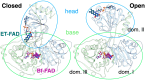
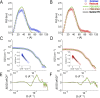
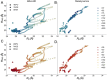
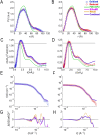
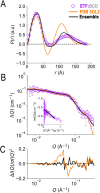
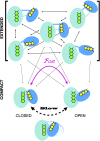
Electron transfer bifurcation enables biological systems to drive unfavourable (endergonic) electron transfer by coupling it to favourable (exergonic) transfer of a second electron. In electron transfer flavoproteins (ETFs), a domain-scale conformational change is believed to sever the favourable pathway after a single electron has used it, thereby preventing the energy dissipation that would accompany exergonic transfer of the second electron. To understand the conformation change that participates in turnover, we have deployed small-angle neutron scattering (SANS) and computational techniques to characterize the bifurcating ETF from Acidaminococcus fermentans (AfeETF). SANS data reveal an overall radius of gyration (R g) of 30.1 ± 0.2 Å and a maximum dimension (D max) of 100 Å for oxidized AfeETF. These measurements are 4 Å and 30 Å larger, respectively, than those of any published bifurcating ETF structure. Thus, we find that none of the reported ETF structures can explain the observed scattering, nor can any individual conformation generated by either of our molecular dynamics protocols. To optimize ensembles best able to explain the SANS data, we adapted a genetic algorithm. Successful ensembles contained a compact conformation comparable to one of the crystallographically documented conformations, accompanied by a much more extended one, and these two conformations sufficed to account for the data. The extended conformations identified all have R gs at least 4 Å larger than those of any currently published ETF structures. However, they are strongly populated, constituting 20% of the population of reduced ETF and over 50% of the population of oxidized AfeETF. Thus, the published (compact) structures provide a seriously incomplete picture of the conformation of AfeETF in solution. Moreover, because the composition of the conformational ensemble changes upon reduction of AfeETF's flavins, interconversion of the conformations may contribute to turnover. We propose that the extended conformations can provide energetically accessible paths for rapid interconversion of the open and closed compact conformations that are believed essential at alternating points in turnover.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Defining Electron Bifurcation in the Electron-Transferring Flavoprotein Family.J Bacteriol. 2017 Oct 3;199(21):e00440-17. doi: 10.1128/JB.00440-17. Print 2017 Nov 1. J Bacteriol. 2017. PMID: 28808132 Free PMC article.
-
Rapid kinetics reveal surprising flavin chemistry in bifurcating electron transfer flavoprotein from Acidaminococcus fermentans.J Biol Chem. 2021 Jan-Jun;296:100124. doi: 10.1074/jbc.RA120.016017. Epub 2020 Dec 2. J Biol Chem. 2021. PMID: 33239361 Free PMC article.
-
Studies on the mechanism of electron bifurcation catalyzed by electron transferring flavoprotein (Etf) and butyryl-CoA dehydrogenase (Bcd) of Acidaminococcus fermentans.J Biol Chem. 2014 Feb 21;289(8):5145-57. doi: 10.1074/jbc.M113.521013. Epub 2013 Dec 30. J Biol Chem. 2014. PMID: 24379410 Free PMC article.
-
Flavin radicals, conformational sampling and robust design principles in interprotein electron transfer: the trimethylamine dehydrogenase-electron-transferring flavoprotein complex.Biochem Soc Symp. 2004;(71):1-14. doi: 10.1042/bss0710001. Biochem Soc Symp. 2004. PMID: 15777008 Review.
-
Energy conservation via electron bifurcating ferredoxin reduction and proton/Na(+) translocating ferredoxin oxidation.Biochim Biophys Acta. 2013 Feb;1827(2):94-113. doi: 10.1016/j.bbabio.2012.07.002. Epub 2012 Jul 16. Biochim Biophys Acta. 2013. PMID: 22800682 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

