Generation and capture of naphthoquinonynes: a new frontier in the development of trypanocidal quinones via aryne chemistry
- PMID: 39512946
- PMCID: PMC11539365
- DOI: 10.1039/d4md00558a
Generation and capture of naphthoquinonynes: a new frontier in the development of trypanocidal quinones via aryne chemistry
Abstract
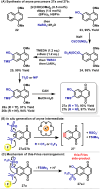
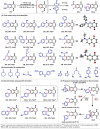
The regioselective synthesis of functionalized naphthoquinones via the formation and capture of naphthoquinonynes has been used to prepare trypanocidal compounds. The target compounds are functionalized on the aromatic ring, leaving the quinoidal ring intact. Using this technique, eighteen functionalized naphthoquinones were succesfull obtained, divided in two main groups: the first scope using N-nucleophiles, and the second scope using pyridine N-oxides, with yields up to 74%. Evaluation against bloodstream trypomastigotes of T. cruzi has identified fourteen compounds that are more potent than benznidazole (Bz); for instance, compounds 29b-I and 30b, with IC50/24 h values of 10.5 and 10.1 μM, respectively, are approximately 10-fold more active than Bz. This study provides the first examples of the application of naphthoquinonyne chemistry for the synthesis of new compounds with potent trypanocidal activities.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Rhodium-catalyzed C-H bond activation for the synthesis of quinonoid compounds: Significant Anti-Trypanosoma cruzi activities and electrochemical studies of functionalized quinones.Eur J Med Chem. 2017 Aug 18;136:406-419. doi: 10.1016/j.ejmech.2017.05.011. Epub 2017 May 4. Eur J Med Chem. 2017. PMID: 28521262
-
The Synthesis and Reactivity of Naphthoquinonynes.Angew Chem Int Ed Engl. 2024 Apr 24;63(18):e202400188. doi: 10.1002/anie.202400188. Epub 2024 Mar 25. Angew Chem Int Ed Engl. 2024. PMID: 38445547
-
Strategies towards potent trypanocidal drugs: Application of Rh-catalyzed [2 + 2 + 2] cycloadditions, sulfonyl phthalide annulation and nitroalkene reactions for the synthesis of substituted quinones and their evaluation against Trypanosoma cruzi.Bioorg Med Chem. 2020 Aug 1;28(15):115565. doi: 10.1016/j.bmc.2020.115565. Epub 2020 May 30. Bioorg Med Chem. 2020. PMID: 32631558
-
Natural and synthetic naphthoquinones active against Trypanosoma cruzi: an initial step towards new drugs for Chagas disease.Curr Med Chem. 2011;18(1):144-61. doi: 10.2174/092986711793979779. Curr Med Chem. 2011. PMID: 21110810 Review.
-
The trypanocidal activity of naphthoquinones: a review.Molecules. 2009 Nov 10;14(11):4570-90. doi: 10.3390/molecules14114570. Molecules. 2009. PMID: 19924086 Free PMC article. Review.
References
-
- Tuddenham S. Hamill M. M. Ghanem K. G. JAMA, J. Am. Med. Assoc. 2022;327:161–172. doi: 10.1001/jama.2021.23487. - DOI - PubMed
- Low N. Broutet N. Adu-Sarkodie Y. Barton P. Hossain M. Hawkes S. Lancet. 2006;368:2001–2016. doi: 10.1016/S0140-6736(06)69482-8. - DOI - PubMed
- Unemo M. Bradshaw C. S. Hocking J. S. de Vries H. J. C. Francis S. C. Mabey D. Marazzo J. M. Sonder G. J. B. Schwebke J. R. Hoornenborg E. Peeling R. W. Philip S. S. Low N. Fairley C. K. Lancet. 2017;17:E235–E279. doi: 10.1016/S1473-3099(17)30310-9. - DOI - PubMed
-
- Kadam S. B. Sukramani G. S. Bishnoi P. Pable A. A. Barvkar V. T. J. Basic Microbiol. 2021;61:180–202. doi: 10.1002/jobm.202000537. - DOI - PMC - PubMed
- Fontanet A. Autran B. Lina B. Kieny M. P. Karim S. S. A. Sridhar D. Lancet. 2021;397:952–954. doi: 10.1016/S0140-6736(21)00370-6. - DOI - PMC - PubMed
- Abduljalil J. M. Abduljalil B. M. New Microbes New Infect. 2020;35:100672. doi: 10.1016/j.nmni.2020.100672. - DOI - PMC - PubMed
- Santos L. H. Kronenberger T. Almeida R. G. Silva E. B. Rocha R. E. O. Oliveira J. C. Barreto L. V. Skinner D. Fajtová P. Giardini M. A. Woodworth B. Bardine C. Lourenço A. L. Craik C. S. Poso A. Podust L. M. McKerrow J. H. Siqueira-Neto J. L. O'Donoghue A. J. da Silva Júnior E. N. Ferreira R. S. J. Chem. Inf. Model. 2022;62:6553–6573. doi: 10.1021/acs.jcim.2c00693. - DOI - PubMed
LinkOut - more resources
Full Text Sources

