Short-Term Efficacy and Safety of Suvorexant and Lemborexant: A Retrospective Study
- PMID: 39512953
- PMCID: PMC11541064
- DOI: 10.7759/cureus.71049
Short-Term Efficacy and Safety of Suvorexant and Lemborexant: A Retrospective Study
Abstract
Purpose: Given the risks of long-term benzodiazepine use, safer alternatives like orexin receptor antagonists (ORAs) are needed for insomnia treatment. This study aims to compare suvorexant and lemborexant, focusing on early-stage sleep duration as an efficacy measure and fall incidence as a safety measure.
Methods: We included patients admitted to our hospital between April 1, 2022 and December 31, 2022, who were newly prescribed suvorexant or lemborexant, excluding patients taking other concomitant sleep medications or antipsychotics. Primary and secondary endpoints were sleep duration during the first three days after taking the medications and the incidence of falls, respectively.
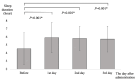
Results: We analyzed data from 48 and 57 patients taking suvorexant and lemborexant, respectively. When compared with that in the pre-treatment period, sleep duration was significantly longer on days 2 and 3 in the suvorexant group, and on all three days in the lemborexant group. On day 1 of drug administration, the lemborexant group had a significantly longer sleep duration than the suvorexant group (5.10 ± 1.84 vs. 5.93 ± 1.90 h, respectively; P = 0.017). Zero (0.0%) and three (5.3%) falls occurred in the suvorexant and lemborexant groups, respectively (P = 0.248).
Conclusions: Lemborexant exerted a potent inhibitory effect on orexin 2 receptors, which could explain the longer sleep duration experienced by patients taking this drug on the first day of treatment.
Keywords: falls; lemborexant; orexin; sleep duration; suvorexant.
Copyright © 2024, Mori et al.
Conflict of interest statement
Human subjects: Consent was obtained or waived by all participants in this study. Institutional Review Board of Ogaki Municipal Hospital, Ogaki, Japan issued approval 20230427-10. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures


References
-
- Delivering cognitive behavioral therapy for insomnia in the real world: considerations and controversies. Buenaver LF, Townsend D, Ong JC. Sleep Med Clin. 2019;14:275–281. - PubMed
-
- Fall-risk-increasing drugs: a systematic review and meta-analysis: II. Psychotropics. Seppala LJ, Wermelink AM, de Vries M, Ploegmakers KJ, van de Glind EM, Daams JG, van der Velde N. J Am Med Dir Assoc. 2018;19:371–377. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
