Efficacy and Safety of Auranofin for Progressive Desmoid-Type Fibromatosis: The Study Protocol of an Open-Label Phase II Trial
- PMID: 39512976
- PMCID: PMC11540592
- DOI: 10.7759/cureus.71033
Efficacy and Safety of Auranofin for Progressive Desmoid-Type Fibromatosis: The Study Protocol of an Open-Label Phase II Trial
Abstract
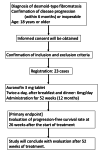
Background As desmoid-type fibromatosis (DF) exhibits a high recurrence rate after surgery, initial active surveillance followed by medical therapy is the mainstay of the treatment. However, there are few effective drugs with acceptable side effects. Methodology Among drugs that have been used for a long period and possess a known safety profile, auranofin was observed to be effective in suppressing DF using the drug repositioning method in our laboratory. This clinical study has been designed to examine the efficacy and safety of auranofin, an approved anti-rheumatic drug, in patients with progressive DF. Results This study is conducted as a single-center, single-arm, open-label study. Auranofin 3 mg tablets will be administered twice daily to DF patients with progressive disease. The primary endpoint is progression-free survival at 26 weeks after starting treatment. Secondary endpoints include response rate, T2-weighted MRI evaluation, pain intensity, quality of life (QOL), and safety assessment. Conclusions This is the first clinical trial of auranofin in patients with aggressive DF. The study will allow an in-depth understanding of the efficacy of auranofin for response rate as well as for changes in MRI findings, pain, and QOL in patients with aggressive DF.
Keywords: auranofin; clinical trial; desmoid; drug repositioning; protocol.
Copyright © 2024, Nishida et al.
Conflict of interest statement
Human subjects: All authors have confirmed that this study did not involve human participants or tissue. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: Yoshihiro Nishida declare(s) a grant from Nagoya University Hospital. This research is supported by Nagoya University Hospital Funding for Clinical Development. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Similar articles
-
Efficacy of auranofin as an inhibitor of desmoid progression.Sci Rep. 2022 Jul 13;12(1):11918. doi: 10.1038/s41598-022-15756-9. Sci Rep. 2022. PMID: 35831372 Free PMC article.
-
The Activity and Safety of Anlotinib for Patients with Extremity Desmoid Fibromatosis: A Retrospective Study in a Single Institution.Drug Des Devel Ther. 2020 Sep 25;14:3941-3950. doi: 10.2147/DDDT.S271008. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 33061299 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Desmoid-type fibromatosis: imaging features and course.Skeletal Radiol. 2023 Jul;52(7):1293-1303. doi: 10.1007/s00256-023-04275-x. Epub 2023 Jan 16. Skeletal Radiol. 2023. PMID: 36646850 Review.
-
Efficacy and safety of cyclooxygenase 2 inhibitors for desmoid tumor management: a systematic review.Nagoya J Med Sci. 2021 Nov;83(4):673-681. doi: 10.18999/nagjms.83.4.673. Nagoya J Med Sci. 2021. PMID: 34916712 Free PMC article.
References
-
- WHO Classification of Tumours Editorial Board. Lyon: IARC Press; 2020. WHO Classification of Tumours Soft Tissue and Bone Tumours.
-
- The desmoid tumor. I. Incidence, sex-, age- and anatomical distribution in the Finnish population. Reitamo JJ, Häyry P, Nykyri E, Saxén E. Am J Clin Pathol. 1982;77:665–673. - PubMed
-
- The management of desmoid tumours: a joint global consensus-based guideline approach for adult and paediatric patients. Eur J Cancer. 2020;127:96–107. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous