Remote-Controlled Gene Delivery in Coaxial 3D-Bioprinted Constructs using Ultrasound-Responsive Bioinks
- PMID: 39513003
- PMCID: PMC11538209
- DOI: 10.1007/s12195-024-00818-x
Remote-Controlled Gene Delivery in Coaxial 3D-Bioprinted Constructs using Ultrasound-Responsive Bioinks
Abstract
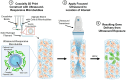
Introduction: Coaxial 3D bioprinting has advanced the formation of tissue constructs that recapitulate key architectures and biophysical parameters for in-vitro disease modeling and tissue-engineered therapies. Controlling gene expression within these structures is critical for modulating cell signaling and probing cell behavior. However, current transfection strategies are limited in spatiotemporal control because dense 3D scaffolds hinder diffusion of traditional vectors. To address this, we developed a coaxial extrusion 3D bioprinting technique using ultrasound-responsive gene delivery bioinks. These bioink materials incorporate echogenic microbubble gene delivery particles that upon ultrasound exposure can sonoporate cells within the construct, facilitating controllable transfection.
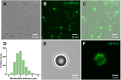
Methods: Phospholipid-coated gas-core microbubbles were electrostatically coupled to reporter transgene plasmid payloads and incorporated into cell-laden alginate bioinks at varying particle concentrations. These bioinks were loaded into the coaxial nozzle core for extrusion bioprinting with CaCl2 crosslinker in the outer sheath. Resulting bioprints were exposed to 2.25 MHz focused ultrasound and evaluated for microbubble activation and subsequent DNA delivery and transgene expression.
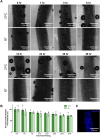
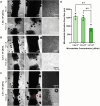
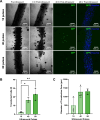
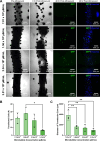
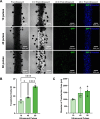
Results: Coaxial printing parameters were established that preserved the stability of ultrasound-responsive gene delivery particles for at least 48 h in bioprinted alginate filaments while maintaining high cell viability. Successful sonoporation of embedded cells resulted in DNA delivery and robust ultrasound-controlled transgene expression. The number of transfected cells was modulated by varying the number of focused ultrasound pulses applied. The size region over which DNA was delivered was modulated by varying the concentration of microbubbles in the printed filaments.
Conclusions: Our results present a successful coaxial 3D bioprinting technique designed to facilitate ultrasound-controlled gene delivery. This platform enables remote, spatiotemporally-defined genetic manipulation in coaxially bioprinted tissue constructs with important applications for disease modeling and regenerative medicine.
Supplementary information: The online version contains supplementary material available at 10.1007/s12195-024-00818-x.
Keywords: Bioink; Biomaterials; Coaxial 3D bioprinting; Controlled delivery; Focused ultrasound; Gene delivery; Microbubbles; Sonoporation; Ultrasound.
© The Author(s) 2024.
Conflict of interest statement
Conflict of interestThe authors MKL, HD, KJS, KTH, CMF and CES declare no conflicts of interest.
Figures


References
-
- Debnath, J., S. K. Muthuswamy, and J. S. Brugge. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 30:256–268, 2003. - PubMed
-
- Alemany-Ribes, M., and C. E. Semino. Bioengineering 3D environments for cancer models. Adv. Drug Deliv. Rev. 79–80:40–49, 2014. - PubMed
-
- Langley, R. R., and I. J. Fidler. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr. Rev. 28:297–321, 2007. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

