Multicompartmentalized Microvascularized Tumor-on-a-Chip to Study Tumor-Stroma Interactions and Drug Resistance in Ovarian Cancer
- PMID: 39513004
- PMCID: PMC11538101
- DOI: 10.1007/s12195-024-00817-y
Multicompartmentalized Microvascularized Tumor-on-a-Chip to Study Tumor-Stroma Interactions and Drug Resistance in Ovarian Cancer
Abstract
Introduction: The majority of ovarian cancer (OC) patients receiving standard of care chemotherapy develop chemoresistance within 5 years. The tumor microenvironment (TME) is a dynamic and influential player in disease progression and therapeutic response. However, there is a lack of models that allow us to elucidate the compartmentalized nature of TME in a controllable, yet physiologically relevant manner and its critical role in modulating drug resistance.
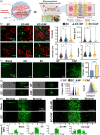
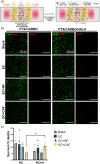
Methods: We developed a 3D microvascularized multiniche tumor-on-a-chip formed by five chambers (central cancer chamber, flanked by two lateral stromal chambers and two external circulation chambers) to recapitulate OC-TME compartmentalization and study its influence on drug resistance. Stromal chambers included endothelial cells alone or cocultured with normal fibroblasts or cancer-associated fibroblasts (CAF).
Results: The tumor-on-a-chip recapitulated spatial TME compartmentalization including vessel-like structure, stromal-mediated extracellular matrix (ECM) remodeling, generation of oxygen gradients, and delayed drug diffusion/penetration from the circulation chamber towards the cancer chamber. The cancer chamber mimicked metastasis-like migration and increased drug resistance to carboplatin/paclitaxel treatment in the presence of CAF when compared to normal fibroblasts. CAF-mediated drug resistance was rescued by ECM targeted therapy. Critically, these results demonstrate that cellular crosstalk recreation and spatial organization through compartmentalization are essential to determining the effect of the compartmentalized OC-TME on drug resistance.
Conclusions: Our results present a functionally characterized microvascularized multiniche tumor-on-a-chip able to recapitulate TME compartmentalization influencing drug resistance. This technology holds the potential to guide the design of more effective and targeted therapeutic strategies to overcome chemoresistance in OC.
Supplementary information: The online version contains supplementary material available at 10.1007/s12195-024-00817-y.
Keywords: Cancer-associated fibroblasts; Compartmentalization; Drug resistance; Ovarian cancer; Tumor microenvironment; Tumor-on-a-chip.
© The Author(s) 2024.
Conflict of interest statement
Conflict of interestDr. Pilar de la Puente and Kristin Calar have a patent for the 3D culture method described in this manuscript, US Patent Application #2022/0228124. Pilar de la Puente is the co-founder of Cellatrix LLC; however, there has been no contribution of the aforementioned entity to the current study. Simona Plesselova, Hailey Axemaker, Emma Sahly, Amrita Bhagia, Jessica Faragher, and Darci Fink state no conflicts of interest.
Figures





Update of
-
Multicompartmentalized microvascularized tumor-on-a-chip to study tumor-stroma interactions and drug resistance in ovarian cancer.bioRxiv [Preprint]. 2024 Jun 2:2024.05.29.596456. doi: 10.1101/2024.05.29.596456. bioRxiv. 2024. Update in: Cell Mol Bioeng. 2024 Sep 14;17(5):345-367. doi: 10.1007/s12195-024-00817-y. PMID: 38853974 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources

