Current Developments in NK Cell Engagers for Cancer Immunotherapy: Focus on CD16A and NKp46
- PMID: 39513028
- PMCID: PMC11538608
- DOI: 10.4110/in.2024.24.e34
Current Developments in NK Cell Engagers for Cancer Immunotherapy: Focus on CD16A and NKp46
Abstract
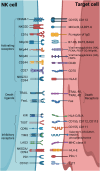
NK cells are specialized immune effector cells crucial for triggering immune responses against aberrant cells. Although recent advancements have concentrated on creating or releasing T-cell responses specific to tumor Ags, the clinical advantages of this approach have been limited to certain groups of patients and tumor types. This emphasizes the need for alternative strategies. One pioneering approach involves broadening and enhancing anti-tumor immune responses by targeting innate immunity. Consequently, the advent of bi-, tri-, and multi-specific Abs has facilitated the advancement of targeted cancer immunotherapies by redirecting immune effector cells to eradicate tumor cells. These Abs enable the simultaneous binding of surface Ags on tumor cells and the activation of receptors on innate immune cells, such as NK cells, with the ability to facilitate Ab-dependent cellular cytotoxicity to enhance their immunotherapeutic effectiveness in patients with solid tumors. Here, we review the recent advances in NK cell engagers (NKCEs) focusing on NK cell-activating receptors CD16A and NKp46. In addition, we provide an overview of the ongoing clinical trials investigating the safety, efficacy, and potential of NKCEs.
Keywords: Antibody-dependent cell cytotoxicity; Immunotherapy; NK cell receptors; NK cells.
Copyright © 2024. The Korean Association of Immunologists.
Conflict of interest statement
Conflict of Interest: The authors declare no potential conflicts of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

