DOK1 facilitates the advancement of ccRCC
- PMID: 39513119
- PMCID: PMC11540502
- DOI: 10.7150/jca.104375
DOK1 facilitates the advancement of ccRCC
Abstract
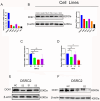
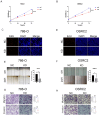
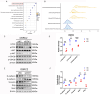
Background: Renal cell carcinoma (RCC) is one of the most common human cancers. Clear cell renal cell carcinoma (ccRCC) is a major subtype of RCC. However, the molecular mechanisms underlying ccRCC oncogenesis require further investigation. Docking protein 1 (DOK1) is a putative tumor suppressor gene; however, its role in ccRCC remains unclear. Methods: Bioinformatic analysis was used to illustrate the poor prognosis associated with DOK1 expression and its role in tumor development in ccRCC in patients. qPCR (quantitative polymerase chain reaction) and western blotting assays were used to validate DOK1 expression in ccRCC cells. In vitro experiments were performed to further elucidate the biological role of DOK1 in ccRCC. Results: DOK1 was overexpressed in ccRCC tissues and cells at both mRNA and protein levels. High DOK1 expression closely correlated with poor survival in patients with ccRCC. DOK1 expression significantly accelerated ccRCC proliferation, migration, invasion, and epithelial-mesenchymal transition (EMT). Through PI3K (phosphatidylin-ositol-3-kinase)/AKT (protein kinase B)/GSK3β (glycogen synthase kinase 3 beta) signaling, DOK1 may control the progression of ccRCC. Conclusion: DOK1 has the potential to serve as a valuable biomarker and target for treatment in ccRCC through its regulation of PI3K/AKT/GSK3β signaling to promote ccRCC progression.
Keywords: DOK1; KIRC; PI3K-AKT; ccRCC; epithelial-mesenchymal transition.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Ericsson JL, Seljelid R, Orrenius S. Comparative light and electron microscopic observations of the cytoplasmic matrix in renal carcinomas. Virchows Arch Pathol Anat Physiol Klin Med. 1966;341:204–23. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

