Navigating investigator-initiated clinical trials: A call for guidelines & monitoring frameworks from an Indian context
- PMID: 39513215
- PMCID: PMC11544576
- DOI: 10.25259/IJMR_353_2024
Navigating investigator-initiated clinical trials: A call for guidelines & monitoring frameworks from an Indian context
Abstract
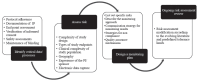
Investigator-initiated clinical trials - also known as non-regulatory or academic clinical trials, are conducted by investigators from academia or research organizations. They usually aim to address scientific questions with insufficient commercial implications and generate real-world applicable solutions, unlike trials sponsored by the pharmaceutical industry which are primarily focused on marketing approval of products that have a commercial value. For the trial results to be credible, adhering to robust methodology and the highest quality standards is paramount. Currently, investigator-initiated clinical trials in India are beyond the purview of the national regulatory authority. They are guided mainly by the National Ethical Guidelines for Biomedical and Health Research Involving Human Participants, 2017 published by Indian Council of Medical Research. They lack an accepted framework for review, conduct, monitoring, reporting of adverse events, and participant compensation. Considering this scenario, we discuss the challenges faced in an investigator initiated clinical trial and explore plausible solutions.
Keywords: Investigator-initiated clinical trial; academic trial; clinical trial monitoring; non-commercial clinical trials; risk-based monitoring.
Conflict of interest statement
None.
Figures
Similar articles
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Interventions to reduce harm from continued tobacco use.Cochrane Database Syst Rev. 2016 Oct 13;10(10):CD005231. doi: 10.1002/14651858.CD005231.pub3. Cochrane Database Syst Rev. 2016. PMID: 27734465 Free PMC article.
References
-
- Central Drugs Standard Control Organisation. Ministry of Health & Family Welfare, Government of India New Drugs & Clinical Trial Rules. 2019. Available from: https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/Pdf-documents/NewDru..., accessed on April 2, 2024.
-
- Enzle ME, Schmaltz R. Ethics review of multi-centre clinical trials in Canada. Health Law Rev. 2005;13:51–7. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous