The role of lipid particle-laden interfaces in regulating the co-delivery of two hydrophobic actives from o/w emulsions
- PMID: 39513340
- PMCID: PMC11552284
- DOI: 10.1080/10717544.2024.2425158
The role of lipid particle-laden interfaces in regulating the co-delivery of two hydrophobic actives from o/w emulsions
Abstract
Co-delivery strategies have become an integral active delivery approach, although understanding of how the microstructural characteristics could be deployed to achieve independently regulated active co-delivery profiles, is still an area at its infancy. Herein, the capacity to provide such control was explored by utilizing Pickering emulsions stabilized by lipid particles, namely solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs). These dual functional species, regarding their concurrent Pickering stabilization and active carrying/delivery capabilities, were formulated with different solid lipid and surfactant types, and the effect on the release and co-release modulation of two hydrophobic actives separately encapsulated within the lipid particles themselves and within the emulsion droplets was investigated. Disparities between the release profiles from the particles in aqueous dispersions or at an emulsion interface, were related to the specific lipid matrix composition. Particles composed of lipids with higher oil phase compatibility of the emulsion droplets were shown to exert less control over their release regulation ability, as were particles in the presence of surfactant micelles in the continuous phase. Irrespective of their formulation characteristics, all particles provided a level of active release control from within the emulsion droplets, which was dependant on the permeability of the formed interfacial layer. Specifically, use of a bulkier particle surfactant or particle sintering at the droplet interface resulted in more sustained droplet release rates. Compared to sole release, the co-release performance remained unaffected by the co-existence of the two hydrophobic actives with the co-release behavior persisting over a storage period of 1 month.
Keywords: Pickering emulsions; Solid lipid nanoparticles; co-encapsulation; co-release; interfacial sintering; nanostructured lipid carriers.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures







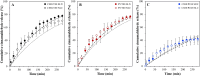



References
-
- Alexandridis P, Holzwarth JF, Hatton TA. (1994). Micellization of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers in aqueous solutions: thermodynamics of copolymer association. Macromolecules 27:2414–25. doi: 10.1021/ma00087a009. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
