Thrombopoietin mimetic reduces mouse lung inflammation and fibrosis after radiation by attenuating activated endothelial phenotypes
- PMID: 39513364
- PMCID: PMC11601560
- DOI: 10.1172/jci.insight.181330
Thrombopoietin mimetic reduces mouse lung inflammation and fibrosis after radiation by attenuating activated endothelial phenotypes
Abstract
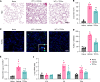
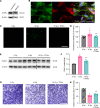
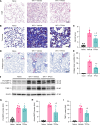
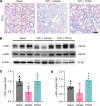
Radiation-induced lung injury (RILI) initiates radiation pneumonitis and progresses to fibrosis as the main side effect experienced by patients with lung cancer treated with radiotherapy. There is no effective drug for RILI. Sustained vascular activation is a major contributor to the establishment of chronic disease. Here, using a whole thoracic irradiation (WTI) mouse model, we investigated the mechanisms and effectiveness of thrombopoietin mimetic (TPOm) for preventing RILI. We demonstrated that administering TPOm 24 hours before irradiation decreased histologic lung injury score, apoptosis, vascular permeability, expression of proinflammatory cytokines, and neutrophil infiltration in the lungs of mice 2 weeks after WTI. We described the expression of c-MPL, a TPO receptor, in mouse primary pulmonary microvascular endothelial cells, showing that TPOm reduced endothelial cell-neutrophil adhesion by inhibiting ICAM-1 expression. Seven months after WTI, TPOm-treated lung exhibited less collagen deposition and expression of MMP-9, TIMP-1, IL-6, TGF-β, and p21. Moreover, TPOm improved lung vascular structure, lung density, and respiration rate, leading to a prolonged survival time after WTI. Single-cell RNA sequencing analysis of lungs 2 weeks after WTI revealed that TPOm shifted populations of capillary endothelial cells toward a less activated and more homeostatic phenotype. Taken together, TPOm is protective for RILI by inhibiting endothelial cell activation.
Keywords: Endothelial cells; Fibrosis; Pulmonology; Radiation therapy; Therapeutics.
Conflict of interest statement
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

