SLC6A19 inhibition facilitates urinary neutral amino acid excretion and lowers plasma phenylalanine
- PMID: 39513367
- PMCID: PMC11601558
- DOI: 10.1172/jci.insight.182876
SLC6A19 inhibition facilitates urinary neutral amino acid excretion and lowers plasma phenylalanine
Abstract
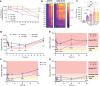
BACKGROUNDThe toxic accumulation of phenylalanine (Phe) in the brain underlies the neurological presentation of phenylketonuria (PKU). Solute carrier family 6 member 19 (SLC6A19) is the major transporter responsible for the (re)absorption of Phe in the kidney and intestine. Here, we describe the characterization of the first small molecule SLC6A19 inhibitor to enter clinical development for the treatment of PKU.METHODSC57Bl/6J WT and Pahenu2 mice were dosed with an inhibitor of SLC6A19 to investigate the effects on urinary amino acids and plasma Phe. In a phase 1 study, healthy human volunteers were dosed with JNT-517, an investigational oral inhibitor of SLC6A19. The primary objective of the study was safety. Secondary objectives included pharmacokinetic and pharmacodynamic studies.RESULTSInhibition of SLC6A19 increased the urinary excretion of Phe in a mouse model of PKU, thereby reducing plasma Phe levels. JNT-517, an investigational oral SLC6A19 inhibitor, was found to be safe and well tolerated and increased the urinary excretion of Phe in a phase 1 healthy volunteer study.CONCLUSIONSThese data indicate that pharmacological inhibition of SLC6A19 presents a promising approach to lower toxic elevated levels of amino acids found in PKU and related amino acid metabolism disorders by facilitating their renal elimination.TRIAL REGISTRATIONAustralian New Zealand Clinical Trials Registry (ANZCTR), ACTRN12622001222730.FUNDINGThe studies in this paper were funded by Jnana Therapeutics.
Keywords: Amino acid metabolism; Clinical trials; Metabolism; Transport.
Figures



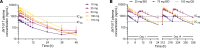

References
-
- Regier DS, Greene CL. Phenylalanine Hydroxylase Deficiency. In: Adam MP, et al, eds. GeneReviews®. University of Washington; 1993.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

