Inflammation associated with monocyte/macrophage activation and recruitment corresponds with lethal outcome in a mouse model of Crimean-Congo haemorrhagic fever1
- PMID: 39513496
- PMCID: PMC11578417
- DOI: 10.1080/22221751.2024.2427782
Inflammation associated with monocyte/macrophage activation and recruitment corresponds with lethal outcome in a mouse model of Crimean-Congo haemorrhagic fever1
Abstract
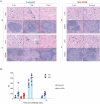
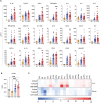
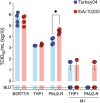
Crimean-Congo haemorrhagic fever virus (CCHFV) causes human disease ranging from subclinical to a fatal haemorrhagic syndrome. Determinants of CCHF pathogenesis are largely unknown and animal models that recapitulate human disease are limited. A recently described mouse model uses a monoclonal antibody (mAb 5A3) targeting the interferon (IFN) alpha/beta receptor to suppress type I IFN responses, making animals transiently susceptible to infection. To advance utility of this model, we investigated effects of challenge route, timing of 5A3 delivery, mouse sex and age, and virus strain on clinical course and outcome. C57BL/6J mice received mAb 5A3 -1, 0, or -1/+1 days post-infection (dpi). Subsets were challenged with CCHFV strain Turkey04 or IbAr10200 subcutaneously or intraperitoneally, and serially euthanized 3- and 7-dpi, when meeting euthanasia criteria or at study completion (14 dpi). CCHFV-IbAr10200-infected mice almost uniformly succumbed to infection, whereas CCHFV-Turkey04-infected mice transiently lost weight but survived. These results were consistent regardless of mAb timing or route of challenge. Viral replication and dissemination were comparable between the two strains at 3 dpi. However, in the plasma and livers of non-survivors, expression of proinflammatory cytokines/chemokines that correspond with macrophage activation and recruitment were significantly elevated. Lethal disease was also associated with elevated levels of macrophage activation marker CD163 in plasma. Further, mouse macrophages were more permissive to IbAr1200 infection in vitro, suggesting tropism for these cells may influence pathogenesis. Our data suggest that early inflammation may be a critical determinant of CCHF outcome and therapeutics to control inflammation may be worthwhile targets for future investigation.
Keywords: CCHF; Crimean-Congo haemorrhagic fever virus; Inflammation; Macrophage activation; Mice; Tropism.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- WHO . WHO – List of Blueprint priority diseases. WHO [Internet]. 2018.
-
- Tasdelen Fisgin N, Doganci L, Tanyel E, et al. . Initial high rate of misdiagnosis in Crimean Congo haemorrhagic fever patients in an endemic region of Turkey. Epidemiol Infect. 2010;138(1):139–144. - PubMed
-
- Swanepoel R, Gill DE, Shepherd AJ, et al. . The clinical pathology of Crimean-Congo hemorrhagic fever. Clinical Infectious Diseases [Internet]. 1989 [cited 2020 Jul 2];11(Supplement_4):S794–S800. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
