Reappraisal of Oncocytic Adenocarcinoma: Unveiling Its Connection to Oncocytic Variants of Salivary Duct Carcinoma and Mucoepidermoid Carcinoma Through ImmunoHisto-Molecular Perspectives
- PMID: 39513520
- PMCID: PMC11634103
- DOI: 10.1097/PAS.0000000000002324
Reappraisal of Oncocytic Adenocarcinoma: Unveiling Its Connection to Oncocytic Variants of Salivary Duct Carcinoma and Mucoepidermoid Carcinoma Through ImmunoHisto-Molecular Perspectives
Abstract
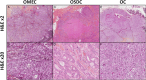
Oncocytic adenocarcinoma (OC) of the salivary glands is a rare and controversial entity. It was recently reclassified as "salivary carcinoma NOS and emerging entities" in the 2022 WHO classification of head and neck tumors. The lack of specific molecular alterations and its potential affiliation with other salivary gland carcinomas, such as the oncocytic mucoepidermoid carcinomas (OMEC) or the oncocytic subtype of salivary duct carcinomas (OSDC) justified this reclassification. It is becoming essential to clarify the complex spectrum of potential diagnoses surrounding oncocytic tumors. The objective of this study was to explore the histologic features, as well as the immunohistochemical and molecular profiles, of cases previously diagnosed as OC or OMEC of the salivary glands. This study involved 28 cases of carcinomas with a predominantly oncocytic component. The sex distribution was equal. The median age was 59 years (range 10 to 89). Most of these cases originated from the parotid gland (25/28). The mean tumor size was 2.4 cm (range 0.5 to 6.5). Primary immuno-morphological and mutation/gene fusion profiles reclassified mainly (64.3%, 18/28). Most of them were reclassified in descending order as OSDC (8/18), OMEC (5/18), and OC (2/18). But 3 cases remained unclassified (3/18). The transcriptomic analysis found a proximity of their transcriptomic profile with the OMEC group and a distance from the OSDCs. These findings imply that OC is not distinct but represents oncocytic variants of other salivary carcinomas. It underscores the importance of thorough morphologic, immunohistochemical, and molecular examinations to accurately diagnose carcinomas with predominant oncocytic components in the salivary glands.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Conflicts of Interest and Source of Funding: This project received funding from HCL, PAM-BIO grants. The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Figures


References
-
- Kaur K, Mehta S, Vanik S, et al. . The evolving role of molecular pathology in the diagnosis of salivary gland tumours with potential pitfalls. Eur Arch Otorhinolaryngol. 2022;279:3769–3783. - PubMed
-
- Skálová A, Stenman G, Simpson RHW, et al. . The role of molecular testing in the differential diagnosis of salivary gland carcinomas. Am J Surg Pathol. 2018;42:e11–e27. - PubMed
-
- Toper MH, Sarioglu S. Molecular pathology of salivary gland neoplasms: diagnostic, prognostic, and predictive perspective. Adv Anat Pathol. 2021;28:81–93. - PubMed
-
- García JJ, Hunt JL, Weinreb I, et al. . Fluorescence in situ hybridization for detection of MAML2 rearrangements in oncocytic mucoepidermoid carcinomas: utility as a diagnostic test. Hum Pathol. 2011;42:2001–2009. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

