The polymeric fluoropyrimidine CF10 overcomes limitations of 5-FU in pancreatic ductal adenocarcinoma cells through increased replication stress
- PMID: 39513592
- PMCID: PMC11552260
- DOI: 10.1080/15384047.2024.2421584
The polymeric fluoropyrimidine CF10 overcomes limitations of 5-FU in pancreatic ductal adenocarcinoma cells through increased replication stress
Abstract
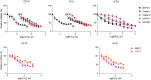
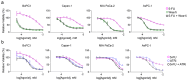
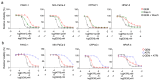
Pancreatic ductal adenocarcinoma (PDAC) is a lethal disease soon to become the second leading cause of cancer deaths in the US. Beside surgery, current therapies have narrow clinical benefits with systemic toxicities. FOLFIRINOX is the current standard of care, one component of which is 5- Fluorouracil (5-FU), which causes serious gastrointestinal and hematopoietic toxicities and is vulnerable to resistance mechanisms. Recently, we have developed polymeric fluoropyrimidines (F10, CF10) which unlike 5-FU, are, in principle, completely converted to the thymidylate synthase inhibitory metabolite FdUMP, without generating appreciable levels of ribonucleotides that cause systemic toxicities while displaying much stronger anti-cancer activity. Here, we confirm the potency of CF10 and investigate enhancement of its efficacy through combination with inhibitors in vitro targeting replication stress, a hallmark of PDAC cells. CF10 is 308-times more potent as a single agent than 5-FU and was effective in the nM range in primary patient derived models. Further, we find that activity of CF10, but not 5-FU, is enhanced through combination with inhibitors of ATR and Wee1 that regulate the S and G2 DNA damage checkpoints and can be reversed by addition of dNTPs indicative of CF10 acting, at least in part, through inducing replication stress. Our results indicate CF10 has the potential to supersede the established benefit of 5-FU in PDAC treatment and indicate novel combination approaches that should be validated in vivo and may be beneficial in established regimens that include 5-FU.
Keywords: DNA damage; PDAC; fluoropyrimidine; pancreatic cancer; replication stress.
Conflict of interest statement
Dr. William H. Gmeiner is an inventor on a patent application related to CF10 treatment of cancer. All other authors declare that they have no conflicts of interest with the contents of this article.
Figures



Similar articles
-
AraC-FdUMP[10] Is a Next-Generation Fluoropyrimidine with Potent Antitumor Activity in PDAC and Synergy with PARG Inhibition.Mol Cancer Res. 2021 Apr;19(4):565-572. doi: 10.1158/1541-7786.MCR-20-0985. Epub 2021 Feb 16. Mol Cancer Res. 2021. PMID: 33593942 Free PMC article.
-
Oncogenic GALNT5 confers FOLFIRINOX resistance via activating the MYH9/ NOTCH/ DDR axis in pancreatic ductal adenocarcinoma.Cell Death Dis. 2024 Oct 21;15(10):767. doi: 10.1038/s41419-024-07110-w. Cell Death Dis. 2024. PMID: 39433745 Free PMC article.
-
The role of DPYD and the effects of DPYD suppressor luteolin combined with 5-FU in pancreatic cancer.Cancer Med. 2024 Aug;13(16):e70124. doi: 10.1002/cam4.70124. Cancer Med. 2024. PMID: 39158384 Free PMC article.
-
Current Standard and Future Perspectives in First- and Second-Line Treatment of Metastatic Pancreatic Adenocarcinoma.Digestion. 2016;94(1):44-9. doi: 10.1159/000447739. Epub 2016 Jul 21. Digestion. 2016. PMID: 27438590 Review.
-
Novel agents for pancreatic ductal adenocarcinoma: emerging therapeutics and future directions.J Hematol Oncol. 2018 Jan 31;11(1):14. doi: 10.1186/s13045-017-0551-7. J Hematol Oncol. 2018. PMID: 29386069 Free PMC article. Review.
References
-
- Kovach JS, Moertel CG, Schutt AJ, Hahn RG, Reitemeier RJ.. Proceedings: a controlled study of combined 1,3-bis-(2-chloroethyl)-1-nitrosourea and 5-fluorouracil therapy for advanced gastric and pancreatic cancer. Cancer. 1974;33:563–10. doi:10.1002/1097-0142(197402)33:2<563:aid-cncr2820330235>3.0.co;2-k. - DOI - PubMed
-
- Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. doi:10.1200/JCO.1997.15.6.2403. - DOI - PubMed
-
- Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias JL, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29(34):4548–4554. doi:10.1200/JCO.2011.36.5742. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
