Compression force promotes glioblastoma progression through the Piezo1‑GDF15‑CTLA4 axis
- PMID: 39513613
- PMCID: PMC11541303
- DOI: 10.3892/or.2024.8835
Compression force promotes glioblastoma progression through the Piezo1‑GDF15‑CTLA4 axis
Abstract
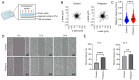
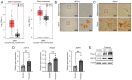
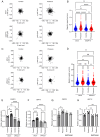
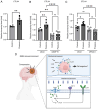
Glioma, a type of brain tumor, is influenced by mechanical forces in its microenvironment that affect cancer progression. However, our understanding of the contribution of compression and its associated mechanisms remains limited. The objective of the present study was to create an environment in which human brain glioma H4 cells experience pressure and thereby investigate the compressive mechanosensors and signaling pathways. Subsequent time‑lapse imaging and wound healing assays confirmed that 12 h of compression significantly increased cell migration, thereby linking compression with enhanced cell motility. Compression upregulated the expression of Piezo1, a mechanosensitive ion channel, and growth differentiation factor 15 (GDF15), a TGF‑β superfamily member. Knockdown experiments targeting PIEZO1 or GDF15 using small interfering RNA resulted in reduced cell motility, with Piezo1 regulating GDF15 expression. Compression also upregulated CTLA4, a critical immune checkpoint protein. The findings of the present study therefore suggest that compression enhances glioma progression by stimulating Piezo1, promoting GDF15 expression and increasing CTLA4 expression. Thus, these findings provide important insights into the influence of mechanical compression on glioma progression and highlight the involvement of the Piezo1‑GDF15 signaling pathway. Understanding tumor responses to mechanical forces in the brain microenvironment may guide the development of targeted therapeutic strategies to mitigate tumor progression and improve patient outcomes.
Keywords: CTLA4; Piezo1; compression force; glioma; growth differentiation factor 15; mechanosensor.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

