Sympathetic Neurons Promote Small Cell Lung Cancer through the β2-Adrenergic Receptor
- PMID: 39513738
- PMCID: PMC11875942
- DOI: 10.1158/2159-8290.CD-24-0718
Sympathetic Neurons Promote Small Cell Lung Cancer through the β2-Adrenergic Receptor
Abstract
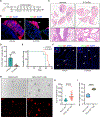
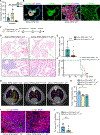
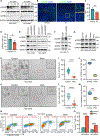
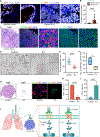
SCLC is highly aggressive, with limited effective treatment options. We show that ablating sympathetic nerves or inhibiting the ADRB2 receptor slows SCLC progression and prolongs survival in mice. Additionally, ADRB2 inhibition reduces the growth of human SCLC organoids and xenografts by disrupting PKA signaling, identifying a new therapeutic target.
©2024 American Association for Cancer Research.
Conflict of interest statement
Author’s Disclosures
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

