Investigating Charge-Induced Transformations of Metal Nanoparticles in a Radically-Inert Liquid: A Liquid-Cell TEM Study
- PMID: 39513789
- PMCID: PMC11547474
- DOI: 10.3390/nano14211709
Investigating Charge-Induced Transformations of Metal Nanoparticles in a Radically-Inert Liquid: A Liquid-Cell TEM Study
Abstract
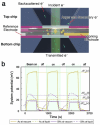
We present a novel in situ liquid-cell transmission electron microscopy (TEM) approach to study the behavior of metal nanoparticles under high-energy electron irradiation. By utilizing a radically-inert liquid environment, we aim to minimize radiolysis effects and explore the influence of charge-induced transformations. We observed complex dynamics in nanoparticle behavior, including morphological changes and transitions between amorphous and crystalline states. These transformations are attributed to the delicate interplay between charge accumulation on the nanoparticles and enhanced radiolysis, suggesting a significant role for charge-assisted processes in nanoparticle evolution. Our findings provide valuable insights into the fundamental mechanisms driving nanoparticle behavior at the nanoscale and demonstrate the potential of liquid-cell TEM for studying complex physicochemical processes in controlled environments.
Keywords: Au nanoparticle; liquid-phase transmission electron microscopy; phase transition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Berry J., Elder K.R., Grant M. Melting at Dislocations and Grain Boundaries: A Phase Field Crystal Study. Phys. Rev. B. 2008;77:224114. doi: 10.1103/PhysRevB.77.224114. - DOI
LinkOut - more resources
Full Text Sources

