Structure-Function Correlation in Cobalt-Induced Brain Toxicity
- PMID: 39513872
- PMCID: PMC11545114
- DOI: 10.3390/cells13211765
Structure-Function Correlation in Cobalt-Induced Brain Toxicity
Abstract
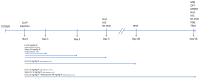
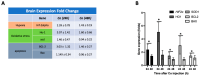
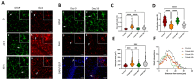
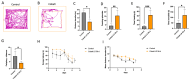
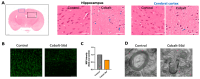
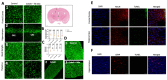
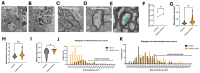
Cobalt toxicity is difficult to detect and therefore often underdiagnosed. The aim of this study was to explore the pathophysiology of cobalt-induced oxidative stress in the brain and its impact on structure and function. Thirty-five wild-type C57B16 mice received intraperitoneal cobalt chloride injections: a single high dose with evaluations at 24, 48, and 72 h (n = 5, each) or daily low doses for 28 (n = 5) or 56 days (n = 15). A part of the 56-day group also received minocycline (n = 5), while 10 mice served as controls. Behavioral changes were evaluated, and cobalt levels in tissues were measured with particle-induced X-ray emission. Brain sections underwent magnetic resonance imaging (MRI), electron microscopy, and histological, immunohistochemical, and molecular analyses. High-dose cobalt caused transient illness, whereas chronic daily low-dose administration led to long-term elevations in cobalt levels accompanied by brain inflammation. Significant neurodegeneration was evidenced by demyelination, increased blood-brain barrier permeability, and mitochondrial dysfunction. Treated mice exhibited extended latency periods in the Morris water maze test and heightened anxiety in the open field test. Minocycline partially mitigated brain injury. The observed signs of neurodegeneration were dose- and time-dependent. The neurotoxicity after acute exposure was reversible, but the neurological and functional changes following chronic cobalt administration were not.
Keywords: MRI; Morris water maze; PIXE; behavioral tests; cobalt; electron microscopy; minocycline; neurodegeneration; open field test.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Kovochich M., Finley B.L., Novick R., Monnot A.D., Donovan E., Unice K.M., Fung E.S., Fung D., Paustenbach D.J. Understanding outcomes and toxicological aspects of second generation metal-on-metal hip implants: A state-of-the-art review. Crit. Rev. Toxicol. 2018;48:853–901. doi: 10.1080/10408444.2018.1563048. - DOI - PubMed
-
- MDA/2017/018. Medical Device Alert. All Metal-on-Metal (MoM) Hip Replacements: Updated Advice for Follow-Up of Patients. [(accessed on 29 November 2021)]; Available online: http://www.mhra.gov.uk/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

