Prognostic Significance of Tumor-Stroma Ratio (TSR) in Head and Neck Squamous Cell Carcinoma: Systematic Review and Meta-Analysis
- PMID: 39513879
- PMCID: PMC11545263
- DOI: 10.3390/cells13211772
Prognostic Significance of Tumor-Stroma Ratio (TSR) in Head and Neck Squamous Cell Carcinoma: Systematic Review and Meta-Analysis
Abstract
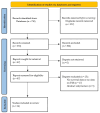
The management of head and neck squamous cell carcinoma (HNSCC) relies heavily on TNM staging and WHO histologic grading; however, in recent years, the analysis of prognostic markers expressed in the tumor stroma has gained attention. The tumor-stroma ratio (TSR) quantifies the proportion of tumor tissue relative to the surrounding stromal tissue; it is assessed with the percentage of stromal tissue within the tumor area, with a cutoff point of 50% being widely used to discriminate high-stroma cancer. In this systematic review and meta-analysis, we investigated the potential prognostic role of the TSR in HNSCC. After a literature screening, 24 studies dealing with the TSR and survival outcomes were included. The TSR showed a significant association with overall survival (OS) in both unadjusted and adjusted measures (RR 2.04, CI 1.57-2.65, p < 0.01; HR 2.36 CI 1.89-2.94, p < 0.00001), with an even stronger prognostic potential in oral cavity/oral tongue cancers (RR 2.44 CI 1.84-3.22, p < 0.00001). The TSR also showed prognostic value when dealing with cancer-specific survival and was associated with a reduction in disease-free survival (DFS). In particular, the TSR also retained its prognostic role in terms of DFS when specifically considering early-stage cancers in both unadjusted and adjusted analyses (RR 1.81 CI 1.57-2.10, p < 0.00001; HR 2.09 CI 1.58-2.76, p < 0.00001). Therefore, we conclude that the TSR is a reliable prognostic marker that is easy to assess in routine histological slides and can be effectively implemented in the routine evaluation of HNSCC.
Keywords: HNSCC; meta-analysis; oral cancer; prognostic markers; systematic review; tumor–stroma ratio.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Prognostic value of tumor-stroma ratio in oral carcinoma: Role of cancer-associated fibroblasts.Oral Dis. 2023 Jul;29(5):1967-1978. doi: 10.1111/odi.14203. Epub 2022 Apr 19. Oral Dis. 2023. PMID: 35388593
-
Clinical significance of tumor-stroma ratio in head and neck cancer: a systematic review and meta-analysis.BMC Cancer. 2021 Apr 30;21(1):480. doi: 10.1186/s12885-021-08222-8. BMC Cancer. 2021. PMID: 33931044 Free PMC article.
-
Prognostic and clinicopathological significance of tumor-stroma ratio in head and neck squamous cell carcinoma: A systematic review.Med Oral Patol Oral Cir Bucal. 2022 Jul 1;27(4):e301-e309. doi: 10.4317/medoral.24922. Med Oral Patol Oral Cir Bucal. 2022. PMID: 35717622 Free PMC article.
-
Tumor-Stroma Ratio in Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis.Oral Dis. 2025 Mar 24. doi: 10.1111/odi.15319. Online ahead of print. Oral Dis. 2025. PMID: 40127161 Review.
-
Prognostication for oral squamous cell carcinoma patients based on the tumour-stroma ratio and tumour budding.Histopathology. 2020 May;76(6):906-918. doi: 10.1111/his.14070. Epub 2020 May 3. Histopathology. 2020. PMID: 31984527
Cited by
-
Tumor-stroma proportion is associated with increased M2 macrophage abundance and predicts the resistance to immune checkpoint blockade in breast cancer.Transl Oncol. 2025 Apr;54:102343. doi: 10.1016/j.tranon.2025.102343. Epub 2025 Mar 10. Transl Oncol. 2025. PMID: 40068383 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

