Stress Granule Assembly in Pulmonary Arterial Hypertension
- PMID: 39513903
- PMCID: PMC11544768
- DOI: 10.3390/cells13211796
Stress Granule Assembly in Pulmonary Arterial Hypertension
Abstract
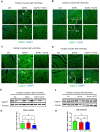
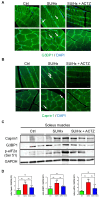
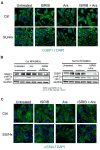
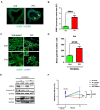
The role of stress granules (SGs) in pulmonary arterial hypertension (PAH) is unknown. We hypothesized that SG formation contributes to abnormal vascular phenotypes, and cardiac and skeletal muscle dysfunction in PAH. Using the rat Sugen/hypoxia (SU/Hx) model of PAH, we demonstrate the formation of SG puncta and increased expression of SG proteins compared to control animals in lungs, right ventricles, and soleus muscles. Acetazolamide (ACTZ) treatment ameliorated the disease and reduced SG formation in all of these tissues. Primary pulmonary artery smooth muscle cells (PASMCs) from diseased animals had increased SG protein expression and SG number after acute oxidative stress and this was ameliorated by ACTZ. Pharmacologic inhibition of SG formation or genetic ablation of the SG assembly protein (G3BP1) altered the SU/Hx-PASMC phenotype by decreasing proliferation, increasing apoptosis and modulating synthetic and contractile marker expression. In human PAH lungs, we found increased SG puncta in pulmonary arteries compared to control lungs and in human PAH-PASMCs we found increased SGs after acute oxidative stress compared to healthy PASMCs. Genetic ablation of G3BP1 in human PAH-PASMCs resulted in a phenotypic switch to a less synthetic and more contractile phenotype. We conclude that increased SG formation in PASMCs and other tissues may contribute to PAH pathogenesis.
Keywords: ACTZ; Caprin1; G3BP1; ISRIB; PAH; stress granules; vascular smooth muscle cells.
Conflict of interest statement
L.E.F. is an employee of Regeneron Pharmaceuticals and holds stock and stock options. Other authors declare no conflicts of interest.
Figures



References
-
- Hudalla H., Michael Z., Christodoulou N., Willis G.R., Fernandez-Gonzalez A., Filatava E.J., Dieffenbach P., Fredenburgh L.E., Stearman R.S., Geraci M.W., et al. Carbonic Anhydrase Inhibition Ameliorates Inflammation and Experimental Pulmonary Hypertension. Am. J. Respir. Cell Mol. Biol. 2019;61:512–524. doi: 10.1165/rcmb.2018-0232OC. - DOI - PMC - PubMed
-
- Kedersha N., Anderson P. Mammalian stress granules and processing bodies. Methods Enzymol. 2007;431:61–81. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

