Expression Profiling Identified TRPM7 and HER2 as Potential Targets for the Combined Treatment of Cancer Cells
- PMID: 39513908
- PMCID: PMC11545334
- DOI: 10.3390/cells13211801
Expression Profiling Identified TRPM7 and HER2 as Potential Targets for the Combined Treatment of Cancer Cells
Abstract
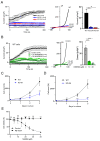
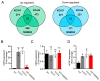
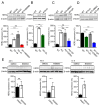
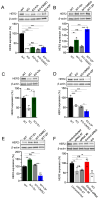
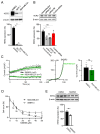
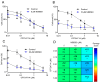
TRPM7 is a divalent cation-permeable channel that is highly active in cancer cells. The pharmacological inhibitors of TRPM7 have been shown to suppress the proliferation of tumor cells, highlighting TRPM7 as a new anticancer drug target. However, the potential benefit of combining TRPM7 inhibitors with conventional anticancer therapies remains unexplored. Here, we used genome-wide transcriptome profiling of human leukemia HAP1 cells to examine cellular responses caused by the application of NS8593, the potent inhibitor of the TRPM7 channel, in comparison with two independent knockout mutations in the TRPM7 gene introduced by the CRISPR/Cas9 approach. This analysis revealed that TRPM7 regulates the expression levels of several transcripts, including HER2 (ERBB2). Consequently, we examined the TRPM7/HER2 axis in several non-hematopoietic cells to show that TRPM7 affects the expression of HER2 protein in a Zn2+-dependent fashion. Moreover, we found that co-administration of pharmacological inhibitors of HER2 and TRPM7 elicited a synergistic antiproliferative effect on HER2-overexpressing SKBR3 cells but not on HER2-deficient MDA-MB-231 breast cancer cells. Hence, our study proposes a new combinatorial strategy for treating HER2-positive breast cancer cells.
Keywords: CP724714; ERBB2; HER2; NS8593; TRP channels; breast cancer; zinc.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Suppression of TRPM7 enhances TRAIL-induced apoptosis in triple-negative breast cancer cells.J Cell Physiol. 2020 Dec;235(12):10037-10050. doi: 10.1002/jcp.29820. Epub 2020 May 29. J Cell Physiol. 2020. PMID: 32468675
-
Carvacrol affects breast cancer cells through TRPM7 mediated cell cycle regulation.Life Sci. 2021 Feb 1;266:118894. doi: 10.1016/j.lfs.2020.118894. Epub 2020 Dec 10. Life Sci. 2021. PMID: 33310045
-
Identification of TG100-115 as a new and potent TRPM7 kinase inhibitor, which suppresses breast cancer cell migration and invasion.Biochim Biophys Acta Gen Subj. 2017 Apr;1861(4):947-957. doi: 10.1016/j.bbagen.2017.01.034. Epub 2017 Feb 1. Biochim Biophys Acta Gen Subj. 2017. PMID: 28161478
-
Mapping TRPM7 Function by NS8593.Int J Mol Sci. 2020 Sep 23;21(19):7017. doi: 10.3390/ijms21197017. Int J Mol Sci. 2020. PMID: 32977698 Free PMC article. Review.
-
Oncogenic role and therapeutic target of transient receptor potential melastatin 7 channel in malignancy.Expert Opin Ther Targets. 2014 Oct;18(10):1177-96. doi: 10.1517/14728222.2014.940894. Epub 2014 Jul 29. Expert Opin Ther Targets. 2014. PMID: 25069584 Review.
Cited by
-
TRPM7 underlies cadmium cytotoxicity in pulmonary cells.Arch Toxicol. 2025 Aug;99(8):3269-3281. doi: 10.1007/s00204-025-04058-4. Epub 2025 May 15. Arch Toxicol. 2025. PMID: 40369100 Free PMC article.
References
-
- Bates-Withers C., Sah R., Clapham D.E. TRPM7, the Mg2+ inhibited channel and kinase. Adv. Exp. Med. Biol. 2011;704:173–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

