Pathogenic Variants in Cancer Susceptibility Genes Predispose to Ductal Carcinoma In Situ of the Breast
- PMID: 39513960
- PMCID: PMC11701432
- DOI: 10.1158/1078-0432.CCR-24-1884
Pathogenic Variants in Cancer Susceptibility Genes Predispose to Ductal Carcinoma In Situ of the Breast
Abstract
Purpose: To determine the relationship between germline pathogenic variants (PV) in cancer predisposition genes and the risk of ductal carcinoma in situ (DCIS).
Experimental design: Germline PV frequencies in breast cancer predisposition genes (ATM, BARD1, BRCA1, BRCA2, CDH1, CHEK2, PALB2, RAD51C, and RAD51D) were compared between DCIS cases and unaffected controls and between DCIS and invasive ductal breast cancer (IDC) cases from a clinical testing cohort (n = 9,887), a population-based cohort (n = 3,876), and the UK Biobank (n = 2,421). The risk of contralateral breast cancer (CBC) for DCIS cases with PV was estimated in the population-based cohort.
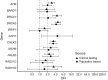
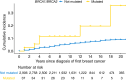
Results: Germline PV were observed in 6.5% and 4.6% of women with DCIS in the clinical testing and population-based cohorts, respectively. BRCA1, BRCA2, and PALB2 PV frequencies were significantly lower among women with DCIS than those with IDC (clinical cohort: 2.8% vs. 5.7%; population-based cohort: 1.7% vs. 3.7%), whereas the PV frequencies for ATM and CHEK2 were similar. ATM, BRCA1, BRCA2, CHEK2, and PALB2 PV were significantly associated with an increased risk of DCIS (OR > 2.0), but only BRCA2 PV were associated with high risk (OR > 4) in both cohorts. The cumulative incidence of CBC among carriers of PV in high-penetrance genes with DCIS was 23% over 15 years.
Conclusions: The enrichment of PV in ATM, BRCA1, BRCA2, CHEK2, and PALB2 among women with DCIS suggests that multigene panel testing may be appropriate for women with DCIS. Elevated risks of CBC in carriers of PV in high-penetrance genes with DCIS confirmed the utility of testing for surgical decision-making.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
R. Karam reports other support from Ambry Genetics outside the submitted work. A. Trentham-Dietz reports grants from NCI during the conduct of the study. A.H. Eliassen reports grants from NIH during the conduct of the study. J.R. Palmer reports grants from NIH during the conduct of the study. C.M. Vachon reports grants from NCI during the conduct of the study, as well as grants from GRAIL outside the submitted work. J.N. Weitzel reports grants from Breast Cancer Research Foundation during the conduct of the study, as well as personal fees from Natera, MyOme, CancerIQ, and AstraZeneca and other support from Natera outside the submitted work. P. Kraft reports grants from NIH during the conduct of the study. J.S. Dolinsky reports personal fees from Ambry Genetics outside the submitted work. M.E. Richardson reports personal fees from Ambry Genetics outside the submitted work. S. Yadav reports other support from AstraZeneca and Repare Therapeutics outside the submitted work. F.J. Couch reports grants from NIH and Breast Cancer Research Foundation during the conduct of the study, as well as grants from GRAIL and personal fees from AstraZeneca outside the submitted work. No disclosures were reported by the other authors.
Figures
References
-
- Ernster VL, Ballard-Barbash R, Barlow WE, Zheng Y, Weaver DL, Cutter G, et al. . Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst 2002;94:1546–54. - PubMed
-
- Claus EB, Stowe M, Carter D. Family history of breast and ovarian cancer and the risk of breast carcinoma in situ. Breast Cancer Res Treat 2003;78:7–15. - PubMed
-
- Kerlikowske K, Barclay J, Grady D, Sickles EA, Ernster V. Comparison of risk factors for ductal carcinoma in situ and invasive breast cancer. J Natl Cancer Inst 1997;89:76–82. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

