Gestational breast cancer: distinctive molecular and clinico-epidemiological features
- PMID: 39514034
- PMCID: PMC11549163
- DOI: 10.1007/s10911-024-09571-3
Gestational breast cancer: distinctive molecular and clinico-epidemiological features
Abstract
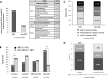
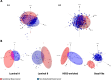
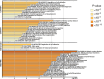
Gestational breast cancer (GBC), defined as breast cancer (BC) diagnosed during pregnancy or the first-year post-partum, accounts for 6-15% of BC cases in women aged 20-44 years. GBC has worse prognosis than non-GBC, but reasons behind are not clear. The GEICAM/2012-03 Study (Molecular Characterization of Gestational Breast Cancer) is a multicenter prospective/retrospective observational registry of patients diagnosed with GBC. From November 2014 to June 2015 seventy patients diagnosed with GBC were included in the study, 30 diagnosed during pregnancy and 40 after delivery. Our current study was aimed to explore differences in epidemiological, clinico-pathological and gene expression features of GBC tumors, from the GEICAM/2012-03 Study, compared to non-GBC tumors from patients of similar age (< 43 years) from six different GEICAM studies, used as non- GBC control population. As per the main objective, the study found multiple differences showing GBC tumors as a different biological entity. GBC showed a more aggressive biology, with higher Ki67 levels, higher incidence of breast and/or ovarian cancer family history, and germline deleterious BRCA1/2 mutations, and are enriched in basal-like intrinsic subtype. GBC patients showed a lower number of tumor infiltrating lymphocytes, while specific genetic signatures highlight differences in GBC´s distinctive transcriptome. Our study shows that GBC is potentially a clinically and molecularly different entity, with specific epidemiological, clinical, and histological features, as well as a distinctive altered immune state and genetic signature. Nevertheless, further studies are needed to better understand the biology of GBC and to identify new targets against which develop new, more effective, targeted therapies.
Keywords: Breast cancer; Gene expression; Gestation; Gestational breast cancer; PAM50 intrinsic subtypes; Tumor infiltrating lymphocytes (TILs).
© 2024. The Author(s).
Conflict of interest statement
J. de la Haba-Rodriguez declares having received research grants from Roche and Pfizer, consulting/advisory fees from AstraZeneca, Amgen, Roche/Genentech, Novartis, Eli Lilly and Pfizer and speakers’ honoraria from AstraZeneca, Lilly, Amgen, Roche/Genentech, Novartis, and Pfizer, but declares no non-financial competing interests. A. Prat declares stock and Other Ownership Interests from Reveal Genomics; honoraria from Pfizer, Novartis, Roche, MSD Oncology, Lilly, Daiichi Sankyo, Amgen, and Guardant Health; consulting/advisory role from Amgen, Roche, Novartis, Pfizer, Bristol-Myers Squibb, Boehringer, PUMA, Oncolytics Biotech., Daiichi Sankyo, Abbvie, AstraZeneca, and NanoString Technologies; research funding from Roche, Novartis, Incyte, and Puma Biotechnology; travel /accommodation expenses from Daiichi Sankyo. Other relationship with companies as, Oncolytics, and Peptomyc S.L.; stock at Reveal Genomics; and patents/royalties from PCT/EP2016/080056, WO/2018/096191, HER2DX filing, and US 63/023785, but declares no non-financial competing interests. A. Guerrero-Zotano declares institutional grant from Pfizer; advisory role honoraria from Novartis, Palex, Pfizer, AstraZeneca and Pierre Fabre; travel grants from Roche, Pfizer, and Novartis, but declares no non-financial competing interests. M. Martin declares having received research grants from Roche, PUMA and Novartis, consulting/advisory fees from AstraZeneca, Amgen, Taiho Oncology, Roche/Genentech, Novartis, PharmaMar, Eli Lilly, PUMA, Taiho Oncology, Daiichi Sankyo and Pfizer and speakers’ honoraria from AstraZeneca, Lilly, Amgen, Roche/Genentech, Novartis, and Pfizer, but declares no non-financial competing interests. F. Rojo declares research grants from Roche, consulting/advisory fees from BMS, MSD, AstraZeneca, Janssen, Roche/Genentech, Novartis, Eli Lilly, and Daiichi Sankyo, but declares no non-financial competing interests. J.A. Perez-Fidalgo declares research grants from Pharmamar, Novartis and GSK consulting/advisory fees from AstraZeneca, Amgen, Abilify Pharma, GSK Tesaro, Pharmamar and Clovis and speakers’ honoraria from AstraZeneca, GSK Tesaro, Pharmamar, Roche, Clovis, and Pfizer. Co-inventorship of a European patent of response prediction in triple negative breast cancer, but declares no non-financial competing interests. J. Gavila declares having received advisory role honoraria from Novartis Pfizer, AstraZeneca; travel grants from Roche, Pfizer, and Novartis, but declares no non-financial competing interests. All other authors declare no financial or non-financial competing interests.
Figures

References
-
- Genin A-S, Lesieur B, Gligorov J, Antoine M, Selleret L, Rouzier R. Pregnancy-associated breast cancers: Do they differ from other breast cancers in young women? The Breast. 2012;21(4):550–5. - PubMed
-
- Langer A, Mohallem M, Stevens D, Rouzier R, Lerebours F, Chérel P. A single-institution study of 117 pregnancy-associated breast cancers (PABC): Presentation, imaging, clinicopathological data and outcome. Diagn Interv Imaging. 2014;95(4):435–41. - PubMed
-
- Pentheroudakis G, Orecchia R, Hoekstra HJ, Pavlidis N. Cancer, fertility and pregnancy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21:v266–73. - PubMed
-
- Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer. 2006;6(4):281–91. - PubMed
-
- Molckovsky A, Madarnas Y. Breast cancer in pregnancy: a literature review. Breast Cancer Res Treat. 2008;108(3):333–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

