System-Wide, Electronic Health Record-Based Medication Alerts for Appropriate Prescribing of Direct Oral Anticoagulants: Pilot Randomized Controlled Trial
- PMID: 39514247
- PMCID: PMC11584537
- DOI: 10.2196/64674
System-Wide, Electronic Health Record-Based Medication Alerts for Appropriate Prescribing of Direct Oral Anticoagulants: Pilot Randomized Controlled Trial
Abstract
Background: While direct oral anticoagulants (DOACs) have improved oral anticoagulation management, inappropriate prescribing remains prevalent and leads to adverse drug events. Antithrombotic stewardship programs seek to enhance DOAC prescribing but require scalable and sustainable strategies.
Objective: We present a pilot, prescriber-level randomized controlled trial to assess the effectiveness of electronic health record (EHR)-based medication alerts in a large health system.
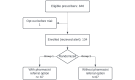
Methods: The pilot assessed prescriber responses to alerts for initial DOAC prescription errors (apixaban and rivaroxaban). A user-centered, multistage design process informed alert development, emphasizing clear indication, appropriate dosing based on renal function, and drug-drug interactions. Alerts appeared whenever a DOAC was being prescribed in a way that did not follow package label instructions. Clinician responses measured acceptability, accuracy, feasibility, and utilization of the alerts.
Results: The study ran from August 1, 2022, through April 30, 2023. Only 1 prescriber requested trial exclusion, demonstrating acceptability. The error rate for false alerts due to incomplete data was 6.6% (16/243). Two scenarios with alert design and/or execution errors occurred but were quickly identified and resolved, underlining the importance of a responsive quality assurance process in EHR-based interventions. Trial feasibility issues related to alert-data capture were identified and resolved. Trial feasibility was also assessed with balanced randomization of prescribers and the inclusion of various alerts across both medications. Assessing utilization, 34.2% (83/243) of the encounters (with 134 prescribers) led to a prescription change.
Conclusions: The pilot implementation study demonstrated the acceptability, accuracy, feasibility, and estimates of the utilization of EHR-based medication alerts for DOAC prescriptions and successfully established just-in-time randomization of prescribing clinicians. This pilot study sets the stage for large-scale, randomized implementation evaluations of EHR-based alerts to improve medication safety.
Trial registration: ClinicalTrials.gov NCT05351749; https://clinicaltrials.gov/study/NCT05351749.
Keywords: EHR; RCT; alert system optimization; clinical decision support; direct oral anticoagulants; electronic health record; medication safety; oral anticoagulants; pilot randomized controlled trial; prescribing errors; randomized controlled trial.
©Shawna N Smith, Michael S M Lanham, F Jacob Seagull, Morris Fabbri, Michael P Dorsch, Kathleen Jennings, Geoffrey Barnes. Originally published in JMIR Formative Research (https://formative.jmir.org), 08.11.2024.
Conflict of interest statement
Conflicts of Interest: SNS provided consulting to Pfizer. MSML is a consultant for the Anticoagulation Forum. GB received grant funding from Boston Scientific; provided consulting to Pfizer, Bristol-Myers Squibb, Janssen, Bayer, AstraZeneca, Sanofi, Anthos, Abbott Vascular, and Boston Scientific; and is on the board of directors of the Anticoagulation Forum. All other authors have no conflicts of interest to disclose.
Figures

References
-
- Stevens SM, Woller SC, Baumann Kreuziger L, Bounameaux H, Doerschug K, Geersing GJ, Huisman M, Kearon C, King CS, Knighton AJ, Lake E, Murin S, Vintch JRE, Wells PS, Moores L. Executive summary: antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021 Dec;160(6):2247–2259. doi: 10.1016/j.chest.2021.07.056.S0012-3692(21)01507-5 - DOI - PubMed
-
- January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019 Jul 09;74(1):104–132. doi: 10.1016/j.jacc.2019.01.011. https://linkinghub.elsevier.com/retrieve/pii/S0735-1097(19)30209-8 S0735-1097(19)30209-8 - DOI - PubMed
-
- Al Rowily A, Jalal Z, Price M, Abutaleb MH, Almodiaemgh H, Al Ammari M, Paudyal V. Prevalence, contributory factors and severity of medication errors associated with direct-acting oral anticoagulants in adult patients: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2022 Apr;78(4):623–645. doi: 10.1007/s00228-021-03212-y. https://europepmc.org/abstract/MED/34935068 10.1007/s00228-021-03212-y - DOI - PMC - PubMed
-
- Budnitz DS, Shehab N, Lovegrove MC, Geller AI, Lind JN, Pollock DA. US emergency department visits attributed to medication harms, 2017-2019. JAMA. 2021 Oct 05;326(13):1299–1309. doi: 10.1001/jama.2021.13844. https://europepmc.org/abstract/MED/34609453 2784662 - DOI - PMC - PubMed
-
- Geller A, Shehab N, Lovegrove M, Weidle N, Budnitz D. Bleeding related to oral anticoagulants: trends in US emergency department visits, 2016-2020. Thromb Res. 2023 May;225:110–115. doi: 10.1016/j.thromres.2023.03.010. https://europepmc.org/abstract/MED/37062120 S0049-3848(23)00084-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

